Ranolazine prevents pressure overload-induced cardiac hypertrophy and heart failure by restoring aberrant Na+ and Ca2+ handling
- PMID: 30488495
- PMCID: PMC6515545
- DOI: 10.1002/jcp.27791
Ranolazine prevents pressure overload-induced cardiac hypertrophy and heart failure by restoring aberrant Na+ and Ca2+ handling
Abstract
Background: Cardiac hypertrophy and heart failure are characterized by increased late sodium current and abnormal Ca2+ handling. Ranolazine, a selective inhibitor of the late sodium current, can reduce sodium accumulation and Ca 2+ overload. In this study, we investigated the effects of ranolazine on pressure overload-induced cardiac hypertrophy and heart failure in mice.
Methods and results: Inhibition of late sodium current with the selective inhibitor ranolazine suppressed cardiac hypertrophy and fibrosis and improved heart function assessed by echocardiography, hemodynamics, and histological analysis in mice exposed to chronic pressure overload induced by transverse aortic constriction (TAC). Ca2+ imaging of ventricular myocytes from TAC mice revealed both abnormal SR Ca 2+ release and increased SR Ca 2+ leak. Ranolazine restored aberrant SR Ca 2+ handling induced by pressure overload. Ranolazine also suppressed Na + overload induced in the failing heart, and restored Na + -induced Ca 2+ overload in an sodium-calcium exchanger (NCX)-dependent manner. Ranolazine suppressed the Ca 2+ -dependent calmodulin (CaM)/CaMKII/myocyte enhancer factor-2 (MEF2) and CaM/CaMKII/calcineurin/nuclear factor of activated T-cells (NFAT) hypertrophy signaling pathways triggered by pressure overload. Pressure overload also prolonged endoplasmic reticulum (ER) stress leading to ER-initiated apoptosis, while inhibition of late sodium current or NCX relieved ER stress and ER-initiated cardiomyocyte apoptosis.
Conclusions: Our study demonstrates that inhibition of late sodium current with ranolazine improves pressure overload-induced cardiac hypertrophy and systolic and diastolic function by restoring Na+ and Ca 2+ handling, inhibiting the downstream hypertrophic pathways and ER stress. Inhibition of late sodium current may provide a new treatment strategy for cardiac hypertrophy and heart failure.
Keywords: Ca2+ transient; Heart failure; INa,L; hypertrophy; ranolazine.
© 2018 Wiley Periodicals, Inc.
Figures


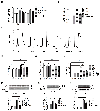
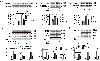
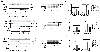

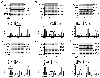
References
-
- Brill DM, and Wasserstrom JA. 1986. Intracellular sodium and the positive inotropic effect of veratridine and cardiac glycoside in sheep Purkinje fibers. Circulation research. 58:109–119. - PubMed
-
- Chaitman BR 2004. Efficacy and safety of a metabolic modulator drug in chronic stable angina: review of evidence from clinical trials. Journal of cardiovascular pharmacology and therapeutics. 9 Suppl 1:S47–64. - PubMed
-
- Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, and Wehrens XH. 2009. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. The Journal of clinical investigation. 119:1940–1951. - PMC - PubMed
-
- Cingolani OH, Yang XP, Cavasin MA, and Carretero OA. 2003. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension. 41:249–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

