Lamin A mutation impairs interaction with nucleoporin NUP155 and disrupts nucleocytoplasmic transport in atrial fibrillation
- PMID: 30488537
- PMCID: PMC6440547
- DOI: 10.1002/humu.23691
Lamin A mutation impairs interaction with nucleoporin NUP155 and disrupts nucleocytoplasmic transport in atrial fibrillation
Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia. Here, we show the identification and functional characterization of one AF-associated mutation p.Arg399Cys in lamin A/C. Co-immunoprecipitation and GST pull-down assays demonstrate that lamin A/C interacts with NUP155, which is a nucleoporin and causes AF when mutated. Lamin A/C mutation p.Arg399Cys impairs the interaction between lamin A/C and NUP155, and increases extractability of NUP155 from the nuclear envelope (NE). Mutation p.Arg399Cys leads to aggregation of lamin A/C in the nucleus, although it does not impair the integrity of NE upon cellular stress. Mutation p.Arg399Cys inhibits the export of HSP70 mRNA and the nuclear import of HSP70 protein. Electrophysiological studies show that mutation p.Arg399Cys decreases the peak cardiac sodium current by decreasing the cell surface expression level of cardiac sodium channel Nav 1.5, but does not affect IKr potassium current. In conclusion, our results indicate that lamin A/C mutation p.Arg399Cys weakens the interaction between nuclear lamina (lamin A/C) and the nuclear pore complex (NUP155), leading to the development of AF. The findings provide a novel molecular mechanism for the pathogenesis of AF.
Keywords: LMNA; NUP155; SCN5A/Nav1.5; atrial fibrillation (AF); mutation.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Figures

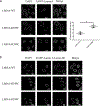


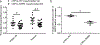
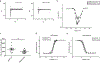
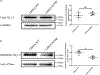
References
-
- Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, & Mohan Rao C (2004). Heat stress-induced localization of small heat shock proteins in mouse myoblasts: Intranuclear lamin A/C speckles as target for alphaB-crystallin and Hsp25. Experimental Cell Research, 299(2), 393–403. 10.1016/j.yexcr.2004.05.032 - DOI - PubMed
-
- Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, … Bonne G (2005). Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Human Molecular Genetics, 14(1), 155–169. 10.1093/hmg/ddi017 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2016 Top-Notch Innovative Talent Development Project from the Bureau of Human Resources and Social Security of Wuhan City/International
- 91439129/National Natural Science Foundation of China/International
- R01 HL126729/HL/NHLBI NIH HHS/United States
- 2012CB517801/Chinese National Basic Research Programs/International
- 81630002/National Natural Science Foundation of China/International
- 81070080/National Natural Science Foundation of China/International
- R01 HL094498/HL/NHLBI NIH HHS/United States
- 2014CFA074/Hubei Province Natural Science Key Program/International
- 31430047/National Natural Science Foundation of China/International
- 81270162/National Natural Science Foundation of China/International
- 2013CB531101/Chinese National Basic Research Programs/International
- 81370206/National Natural Science Foundation of China/International
- 2011ZX09307-001-09/Hubei Province's Outstanding Medical Academic Leader Program Specialized Research Fund for the Doctoral Program of Higher Education from the Ministry of Education, and the "Innovative Development of New Drugs" Key Scientific Project/International
- R01 HL121358/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

