Augmented antitumor activity of 5-fluorouracil by double knockdown of MDM4 and MDM2 in colon and gastric cancer cells
- PMID: 30488540
- PMCID: PMC6361612
- DOI: 10.1111/cas.13893
Augmented antitumor activity of 5-fluorouracil by double knockdown of MDM4 and MDM2 in colon and gastric cancer cells
Abstract
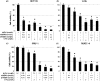
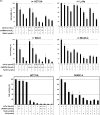
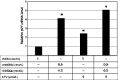
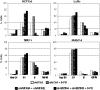
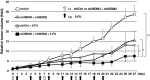
Inactivation of the TP53 tumor suppressor gene is essential during cancer development and progression. Mutations of TP53 are often missense and occur in various human cancers. In some fraction of wild-type (wt) TP53 tumors, p53 is inactivated by upregulated murine double minute homolog 2 (MDM2) and MDM4. We previously reported that simultaneous knockdown of MDM4 and MDM2 using synthetic DNA-modified siRNAs revived p53 activity and synergistically inhibited in vitro cell growth in cancer cells with wt TP53 and high MDM4 expression (wtTP53/highMDM4). In the present study, MDM4/MDM2 double knockdown with the siRNAs enhanced 5-fluorouracil (5-FU)-induced p53 activation, arrested the cell cycle at G1 phase, and potentiated the antitumor effect of 5-FU in wtTP53/highMDM4 human colon (HCT116 and LoVo) and gastric (SNU-1 and NUGC-4) cancer cells. Exposure to 5-FU alone induced MDM2 as well as p21 and PUMA by p53 activation. As p53-MDM2 forms a negative feedback loop, enhancement of the antitumor effect of 5-FU by MDM4/MDM2 double knockdown could be attributed to blocking of the feedback mechanism in addition to direct suppression of these p53 antagonists. Intratumor injection of the MDM4/MDM2 siRNAs suppressed in vivo tumor growth and boosted the antitumor effect of 5-FU in an athymic mouse xenograft model using HCT116 cells. These results suggest that a combination of MDM4/MDM2 knockdown and conventional cytotoxic drugs could be a promising treatment strategy for wtTP53/highMDM4 gastrointestinal cancers.
Keywords: 5-fluorouracil; MDM4; colon cancer; gastric cancer; p53.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures


References
-
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041‐1047. - PubMed
-
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905‐914. - PubMed
-
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938‐2947. - PubMed
-
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23‐30. - PubMed
-
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first‐line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670‐1676. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

