Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis
- PMID: 30488542
- PMCID: PMC6590149
- DOI: 10.1111/all.13685
Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis
Abstract
Background: Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a type 2-mediated inflammatory disease associated with significant clinical, social, and economic burdens and high unmet therapeutic need. Dupilumab, a fully human monoclonal antibody targeting the interleukin-4 receptor α (IL-4Rα) subunit, demonstrated efficacy and acceptable safety in CRSwNP and other type 2 diseases (eg, atopic dermatitis and asthma). We now report the local effects of dupilumab on type 2 inflammatory biomarkers in nasal secretions and nasal polyp tissues of patients with CRSwNP in a randomized, placebo-controlled, phase 2 trial (NCT01920893).
Methods: Cytokines, chemokines, and total immunoglobulin E (IgE) levels were measured using immunoassay techniques in nasal secretions and nasal polyp tissue homogenates of CRSwNP patients receiving dupilumab 300 mg or placebo weekly for 16 weeks.
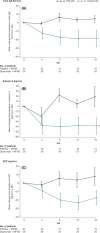
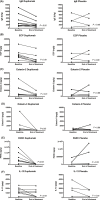
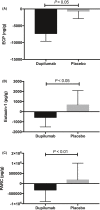
Results: With dupilumab, type 2 biomarker concentrations decreased in nasal secretions (least squares mean area under the curve from 0 to 16 weeks for the change from baseline) vs placebo for eotaxin-3 (-30.06 vs -0.86 pg/mL; P = 0.0008) and total IgE (-7.90 vs -1.86 IU/mL; P = 0.022). Dupilumab treatment also decreased type 2 biomarker levels in nasal polyp tissues at Week 16 vs baseline for eosinophilic cationic protein (P = 0.008), eotaxin-2 (P = 0.008), eotaxin-3 (P = 0.031), pulmonary and activation-regulated chemokine (P = 0.016), IgE (P = 0.023), and IL-13 (P = 0.031).
Conclusions: Dupilumab treatment reduced multiple biomarkers of type 2 inflammation in nasal secretions and polyp tissues of patients with CRSwNP, demonstrating that antagonism of IL-4Rα signaling suppresses IL-4-/IL-13-dependent processes, such as mucosal IgE formation, as well as the expression of chemokines attracting inflammatory cells to the nasal mucosa.
Keywords: biomarkers; dupilumab; nasal polyposis; nasal secretions; type 2 inflammation.
© 2018 The Authors. Allergy Published by John Wiley & Sons Ltd.
Conflict of interest statement
K. Jonstam is the sub‐investigator of the study. B.N. Swanson, L. Mannent L, N. Tian, Y. Wang, D. Zhang, C. Fan, A. Grabher, and G. Pirozzi are employees of and may hold stock and/or stock options in Sanofi. L‐O. Cardell is an associate investigator of the study. G. Holtappels has nothing to disclose. J.D. Hamilton and N.M.H. Graham are employees and shareholders of Regeneron Pharmaceuticals, Inc. C. Bachert is the principal investigator of the study.
Figures
References
-
- Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe – an underestimated disease. A GA2LEN study. Allergy. 2011;66:1216‐1223. - PubMed
-
- Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:743‐12. - PubMed
-
- Tomassen P, Vandeplas G, van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449‐1456. - PubMed
-
- Gröger M, Bernt A, Wolf M, et al. Eosinophils and mast cells: a comparison of nasal mucosa histology and cytology to markers in nasal discharge in patients with chronic sino‐nasal diseases. Eur Arch Otorhinolaryngol. 2013;270:2667‐2676. - PubMed
-
- Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607‐614. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

