Ski drives an acute increase in MMP-9 gene expression and release in primary cardiac myofibroblasts
- PMID: 30488595
- PMCID: PMC6429976
- DOI: 10.14814/phy2.13897
Ski drives an acute increase in MMP-9 gene expression and release in primary cardiac myofibroblasts
Abstract
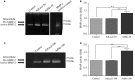
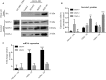
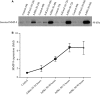
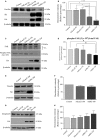
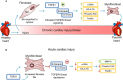
Many etiologies of heart disease are characterized by expansion and remodeling of the cardiac extracellular matrix (ECM or matrix) which results in cardiac fibrosis. Cardiac fibrosis is mediated in cardiac fibroblasts by TGF-β1 /R-Smad2/3 signaling. Matrix component proteins are synthesized by activated resident cardiac fibroblasts known as myofibroblasts (MFB). These events are causal to heart failure with diastolic dysfunction and reduced cardiac filling. We have shown that exogenous Ski, a TGF-β1 /Smad repressor, localizes in the cellular nucleus and deactivates cardiac myofibroblasts. This deactivation is associated with reduction of myofibroblast marker protein expression in vitro, including alpha smooth muscle actin (α-SMA) and extracellular domain-A (ED-A) fibronectin. We hypothesize that Ski also acutely regulates MMP expression in cardiac MFB. While acute Ski overexpression in cardiac MFB in vitro was not associated with any change in intracellular MMP-9 protein expression versus LacZ-treated controls,exogenous Ski caused elevated MMP-9 mRNA expression and increased MMP-9 protein secretion versus controls. Zymographic analysis revealed increased MMP-9-specific gelatinase activity in myofibroblasts overexpressing Ski versus controls. Moreover, Ski expression was attended by reduced paxillin and focal adhesion kinase phosphorylation (FAK - Tyr 397) versus controls. As myofibroblasts are hypersecretory and less motile relative to fibroblasts, Ski's reduction of paxillin and FAK expression may reflect the relative deactivation of myofibroblasts. Thus, in addition to its known antifibrotic effects, Ski overexpression elevates expression and extracellular secretion/release of MMP-9 and thus may facilitate internal cytoskeletal remodeling as well as extracellular ECM components. Further, as acute TGF-β1 treatment of primary cardiac MFB is known to cause rapid translocation of Ski to the nucleus, our data support an autoregulatory role for Ski in mediating cardiac ECM accumulation.
Keywords: Cardiac fibroblast; MMP9; Ski; cardiac fibrosis; cell migration; fibroblast activation; myofibroblast.
© 2018 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Akiyoshi, S. , Inoue H., Hanai J., Kusanagi K., Nemoto N., Miyazono K., et al. 1999. c‐Ski acts as a transcriptional co‐repressor in transforming growth factor‐beta signaling through interaction with smads. J. Biol. Chem. 274:35269–35277. - PubMed
-
- Castro, M. M. , Cena J., Cho W. J., Walsh M. P., and Schulz R.. 2012. Matrix metalloproteinase‐2 proteolysis of calponin‐1 contributes to vascular hypocontractility in endotoxemic rats. Arterioscler. Thromb. Vasc. Biol. 32:662–668. - PubMed
-
- Clutterbuck, A. L. , Asplin K. E., Harris P., Allaway D., and Mobasheri A.. 2009. Targeting matrix metalloproteinases in inflammatory conditions. Curr. Drug Targets 10:1245–1254. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

