The COMiT'ID Study: Developing Core Outcome Domains Sets for Clinical Trials of Sound-, Psychology-, and Pharmacology-Based Interventions for Chronic Subjective Tinnitus in Adults
- PMID: 30488765
- PMCID: PMC6277759
- DOI: 10.1177/2331216518814384
The COMiT'ID Study: Developing Core Outcome Domains Sets for Clinical Trials of Sound-, Psychology-, and Pharmacology-Based Interventions for Chronic Subjective Tinnitus in Adults
Abstract
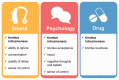
Subjective tinnitus is a chronic heterogeneous condition that is typically managed using intervention approaches based on sound devices, psychologically informed therapies, or pharmaceutical products. For clinical trials, there are currently no common standards for assessing or reporting intervention efficacy. This article reports on the first of two steps to establish a common standard, which identifies what specific tinnitus-related complaints ("outcome domains") are critical and important to assess in all clinical trials to determine whether an intervention has worked. Using purposive sampling, 719 international health-care users with tinnitus, health-care professionals, clinical researchers, commercial representatives, and funders were recruited. Eligibility was primarily determined by experience of one of the three interventions of interest. Following recommended procedures for gaining consensus, three intervention-specific, three-round, Delphi surveys were delivered online. Each Delphi survey was followed by an in-person consensus meeting. Viewpoints and votes involved all stakeholder groups, with approximately a 1:1 ratio of health-care users to professionals. "Tinnitus intrusiveness" was voted in for all three interventions. For sound-based interventions, the minimum set included "ability to ignore," "concentration," "quality of sleep," and "sense of control." For psychology-based interventions, the minimum set included "acceptance of tinnitus," "mood," "negative thoughts and beliefs," and "sense of control." For pharmacology-based interventions, "tinnitus loudness" was the only additional core outcome domain. The second step will next identify how those outcome domains should best be measured. The uptake of these intervention-specific standards in clinical trials will improve research quality, enhance clinical decision-making, and facilitate meta-analysis in systematic reviews.
Keywords: assessment; patient-reported outcome measures; stakeholder agreement; treatment effectiveness.
Figures

References
-
- Baguley D., McFerran D., Hall D. (2013) Tinnitus. The Lancet 382(9904): 1600–1607. doi:10.1016/S0140-6736(13): 60142-7. - PubMed
-
- Chalmers I., Glasziou P. (2009) Avoidable waste in the production and reporting of research evidence. The Lancet 374(9683): 86–89. doi:10.1016/S0140-6736(09)60329-9. - PubMed
-
- De Vet H. C., Terwee C. B., Mokkink L. B., Knol D. L. (2011) Measurement in medicine: A practical guide, New York, NY: Cambridge University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

