Analytical variation in factor VIII one-stage and chromogenic assays: Experiences from the ECAT external quality assessment programme
- PMID: 30488994
- PMCID: PMC6916413
- DOI: 10.1111/hae.13643
Analytical variation in factor VIII one-stage and chromogenic assays: Experiences from the ECAT external quality assessment programme
Abstract
Background: Both one-stage (OSA) and chromogenic substrate assays (CSA) are used to measure factor VIII (FVIII) activity. Factors explaining analytical variation in FVIII activity levels are still to be completely elucidated.
Aim: The aim of this study was to investigate and quantify the analytical variation in OSA and CSA.
Methods: Factors determining analytical variation were studied in sixteen lyophilized plasma samples (FVIII activity <0.01-1.94 IU/mL) and distributed by the ECAT surveys. To elucidate the causes of OSA variation, we exchanged deficient plasma between three company set-ups.
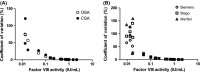
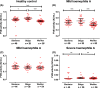
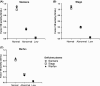
Results: On average, 206 (range 164-230) laboratories used the OSA to measure FVIII activity and 30 (range 12-51) used CSA. The coefficient of variation of OSA and CSA increased with lower FVIII levels (FVIII <0.05 IU/mL). This resulted in misclassification of a severe haemophilia A sample into a moderate or mild haemophilia A sample in 4/30 (13.3%) of CSA measurements, while this was 37/139 (26.6%) for OSA. OSA measurements performed with reagents and equipment from Werfen showed slightly lower FVIII activity (0.93, IQR 0.88-0.98 IU/mL) compared to measurements with Stago (1.07, IQR 1.02-1.14 IU/mL) and Siemens (1.03, IQR 0.97-1.07 IU/mL). Part of this difference is explained by the value of the calibrator. For CSA, the measured FVIII levels were similar using the different kits.
Conclusions: In the lower range (<0.05 IU/mL), analytical variation of FVIII measurements is high in both OSA and CSA measurements. The variation in FVIII activity levels was partly explained by specific manufacturers. Further standardization of FVIII measurements and understanding of analytical variation is required.
Keywords: FVIII measurements; factor VIII; haemophilia A.
© 2018 The Authors. Haemophilia Published by John Wiley & Sons Ltd.
Conflict of interest statement
F.W.G. Leebeek received research support from CSL Behring and Shire for performing the Willebrand in the Netherlands (WiN) study, and is consultant for UniQure, Novo Nordisk and Shire, of which the fees go to the institution. M.H. Cnossen has received unrestricted research/educational and travel funding from the following companies: Pfizer, Baxter, Bayer Schering Pharma, CSL Behring, Novo Nordisk, Novartis and Roche, and serves as a member on steering boards of Roche and Bayer of which fees go to the institution. M.P.M. de Maat is a member of the supervisory board of the ECAT foundation and received unrestricted research/educational funding from Siemens, Werfen and Stago. The remaining authors stated that they had no interests which might be perceived as posing a conflict or bias.
Figures


Similar articles
-
Agreement between one stage and chromogenic assays in samples from patients receiving recombinant porcine FVIII (Obizur, Susoctocog-alfa).Int J Lab Hematol. 2024 Feb;46(1):135-140. doi: 10.1111/ijlh.14181. Epub 2023 Oct 5. Int J Lab Hematol. 2024. PMID: 37799011
-
Factor VIII assay variability in postinfusion samples containing full length and B-domain deleted FVIII.Haemophilia. 2016 Sep;22(5):806-12. doi: 10.1111/hae.12962. Epub 2016 May 24. Haemophilia. 2016. PMID: 27217329
-
Accurate measurement of extended half-life and unmodified factor VIII low levels with one-stage FVIII assays is dependent on the matrix of calibration curves.Haemophilia. 2019 Jan;25(1):e19-e26. doi: 10.1111/hae.13656. Epub 2018 Dec 27. Haemophilia. 2019. PMID: 30589148
-
A critical appraisal of one-stage and chromogenic assays of factor VIII activity.J Thromb Haemost. 2016 Feb;14(2):248-61. doi: 10.1111/jth.13215. Epub 2016 Feb 1. J Thromb Haemost. 2016. PMID: 26663865 Review.
-
One-stage vs. chromogenic assays in haemophilia A.Eur J Haematol. 2015 Feb;94 Suppl 77:38-44. doi: 10.1111/ejh.12500. Eur J Haematol. 2015. PMID: 25560793 Review.
Cited by
-
Area under the curve: Comparing the value of factor VIII replacement therapies in haemophilia A.Haemophilia. 2023 Jan;29(1):145-155. doi: 10.1111/hae.14691. Epub 2022 Nov 29. Haemophilia. 2023. PMID: 36445343 Free PMC article.
-
The traceability of commercial plasma calibrators to the plasma International Standards for factor VIII and factor IX.Int J Lab Hematol. 2020 Dec;42(6):810-818. doi: 10.1111/ijlh.13277. Epub 2020 Jul 8. Int J Lab Hematol. 2020. PMID: 32638532 Free PMC article.
-
International consensus recommendations on the management of people with haemophilia B.Ther Adv Hematol. 2022 Apr 2;13:20406207221085202. doi: 10.1177/20406207221085202. eCollection 2022. Ther Adv Hematol. 2022. PMID: 35392437 Free PMC article. Review.
-
Estimation of Nuwiq® (simoctocog alfa) activity using one-stage and chromogenic assays-Results from an international comparative field study.Haemophilia. 2019 Jul;25(4):708-717. doi: 10.1111/hae.13763. Epub 2019 May 20. Haemophilia. 2019. PMID: 31106957 Free PMC article.
-
Predictive performance of pharmacokinetic-guided prophylactic dosing of factor concentrates in hemophilia A and B.Res Pract Thromb Haemost. 2024 Mar 28;8(3):102397. doi: 10.1016/j.rpth.2024.102397. eCollection 2024 Mar. Res Pract Thromb Haemost. 2024. PMID: 38689619 Free PMC article.
References
-
- Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1‐e47. - PubMed
-
- Leebeek F, Mauser‐Bunschoten EP. Richtlijn Diagnostiek en Behandeling van Hemofilie en Aanverwante Hemostase Stoornissen. Utrecht: Van Zuiden Communications BV; 2009.
-
- Fijnvandraat K, Cnossen MH, Leebeek FW, Peters M. Diagnosis and management of haemophilia. BMJ. 2012;344:e2707. - PubMed
-
- van Moort I, Joosten M, de Maat MP, Leebeek FW, Cnossen MH. Pitfalls in the diagnosis of hemophilia severity: What to do? Pediatr Blood Cancer. 2017;64. - PubMed
-
- Armstrong E, Astermark J, Baghaei F, et al. Nordic hemophilia guidelines. Nordic Hemophilia Council guideline working group. 2015;1‐93.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

