Natural Killer Cell Homing and Persistence in the Bone Marrow After Adoptive Immunotherapy Correlates With Better Leukemia Control
- PMID: 30489431
- PMCID: PMC6365204
- DOI: 10.1097/CJI.0000000000000250
Natural Killer Cell Homing and Persistence in the Bone Marrow After Adoptive Immunotherapy Correlates With Better Leukemia Control
Abstract
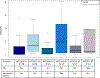
Cellular immunotherapy using allogeneic natural killer (NK) cells may overcome chemotherapy-refractory acute myeloid leukemia. Our goal was to document NK cell homing/persistence in the bone marrow following adoptive immunotherapy. Our cohort included 109 patients who received NK cell therapy for refractory acute myeloid leukemia following lymphodepleting conditioning +/- denileukin diftitox, +/- low-dose total body irradiation. We evaluated the NK cell density in bone marrow core biopsies performed an average of 14 days after NK cell transfer using a CD56 immunohistochemical stain. The NK cell density in core biopsies showed only moderate correlation with NK cell percentage in bone marrow aspirates evaluated by flow cytometry (rs=0.48) suggesting that distribution of CD56 cells in the bone marrow niche offers unique insight into NK cell homing. Better leukemia control was associated with increased NK cell density, such that patients with <5% blasts had a higher NK cell density (P=0.01). As well, NK cell density above the median of reference group was significantly associated with morphologic remission of leukemia (P=0.01). Moreover, the NK cell density varied significantly between conditioning protocols. Our findings suggest that the use of low-dose irradiation or CD25-targeting immunocytokine (denileukin diftitox, IL2DT) as part of conditioning results in increased NK cell homing/persistence in the bone marrow. These novel results will help guide future immunotherapy with NK cells.
Conflict of interest statement
Disclosure of Interest
The authors have no conflict of interest.
Figures
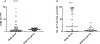


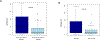

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

