IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils
- PMID: 30496253
- PMCID: PMC6264496
- DOI: 10.1371/journal.pone.0208150
IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils
Abstract
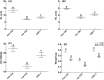
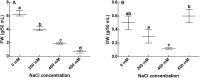
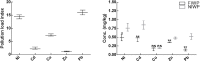
Heavy metals contaminated soil is a serious environmental concern that has a negative impact on agriculture and ecosystem. Economical and efficient ways are needed to address this problem worldwide. In this regard, exploration and application of proficient microbial strains that can help the crop plants to thrive in agricultural soils that are greatly contaminated with heavy metals. The present study mainly focused on the effect of IAA producing endophytic fungi Penicillium ruqueforti Thom., on wheat plants cultivated in soil rich in heavy metals (Ni, Cd, Cu, Zn, and Pb). P. ruqueforti has induced great resistance in wheat inoculated plants grown in heavy metal contaminated soil. Application of the isolated strain of P. ruqueforti restricted the transfer of heavy metals from soil to the plants by secreting indole acetic acid (IAA). Furthermore, P. ruqueforti inoculated wheat seedlings watered with waste water had higher plant growth, nutrient uptake and low concentrations of heavy metals in shoot and roots. On the contrary, non-inoculated wheat plants under heavy metal stress had stunted growth with symptoms of chlorosis. From the results, it is concluded that P. ruqueforti inoculation can establish a symbiotic relationship with host plants, which is useful for phytostabilization of heavy metals or in other words helping the host crops to flourish through soil that are highly contaminated with heavy metals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




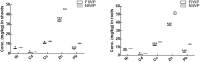

References
-
- Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma G, Sahoo L, et al. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem. 2012;53:33–9. 10.1016/j.plaphy.2012.01.006 - DOI - PubMed
-
- Rizvi A, Khan MS. Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere. 2017;185:942–52. 10.1016/j.chemosphere.2017.07.088 - DOI - PubMed
-
- Balkhair KS, Ashraf MA. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi journal of biological sciences. 2016;23(1):S32–S44. 10.1016/j.sjbs.2015.09.023 - DOI - PMC - PubMed
-
- Abou-Shanab R, Angle J, Chaney R. Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biology and Biochemistry. 2006;38(9):2882–9. 10.1016/j.soilbio.2006.04.045 - DOI
-
- Bradl H. Heavy metals in the environment: origin, interaction and remediation: Academic Press; 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

