Optimizing the immunogenicity of HIV prime-boost DNA-MVA-rgp140/GLA vaccines in a phase II randomized factorial trial design
- PMID: 30496299
- PMCID: PMC6264478
- DOI: 10.1371/journal.pone.0206838
Optimizing the immunogenicity of HIV prime-boost DNA-MVA-rgp140/GLA vaccines in a phase II randomized factorial trial design
Abstract
Background: We evaluated the safety and immunogenicity of (i) an intradermal HIV-DNA regimen given with/without intradermal electroporation (EP) as prime and (ii) the impact of boosting with modified vaccinia virus Ankara (HIV-MVA) administered with or without subtype C CN54rgp140 envelope protein adjuvanted with Glucopyranosyl Lipid A (GLA-AF) in volunteers from Tanzania and Mozambique.
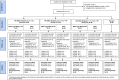
Methods: Healthy HIV-uninfected adults (N = 191) were randomized twice; first to one of three HIV-DNA intradermal priming regimens by needle-free ZetaJet device at weeks 0, 4 and 12 (Group I: 2x0.1mL [3mg/mL], Group II: 2x0.1mL [3mg/mL] plus EP, Group III: 1x0.1mL [6mg/mL] plus EP). Second the same volunteers received 108 pfu HIV-MVA twice, alone or combined with CN54rgp140/GLA-AF, intramuscularly by syringe, 16 weeks apart. Additionally, 20 volunteers received saline placebo.
Results: Vaccinations and electroporation did not raise safety concerns. After the last vaccination, the overall IFN-γ ELISpot response rate to either Gag or Env was 97%. Intradermal electroporation significantly increased ELISpot response rates to HIV-DNA-specific Gag (66% group I vs. 86% group II, p = 0.026), but not to the HIV-MVA vaccine-specific Gag or Env peptide pools nor the magnitude of responses. Co-administration of rgp140/GLA-AF with HIV-MVA did not impact the frequency of binding antibody responses against subtype B gp160, C gp140 or E gp120 antigens (95%, 99%, 79%, respectively), but significantly enhanced the magnitude against subtype B gp160 (2700 versus 300, p<0.001) and subtype C gp140 (24300 versus 2700, p<0.001) Env protein. At relatively low titers, neutralizing antibody responses using the TZM-bl assay were more frequent in vaccinees given adjuvanted protein boost.
Conclusion: Intradermal electroporation increased DNA-induced Gag response rates but did not show an impact on Env-specific responses nor on the magnitude of responses. Co-administration of HIV-MVA with rgp140/GLA-AF significantly enhanced antibody responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- UNAIDS JUN-PoHA. AIDS by the numbers Geneva, Switzerland: 2017.
-
- O'Connell RJ, Kim JH, Corey L, Michael NL. Human immunodeficiency virus vaccine trials. Cold Spring Harb Perspect Med. 2012;2(12):a007351 10.1101/cshperspect.a007351 ; PubMed Central PMCID: PMCPMC3543076. - DOI - PMC - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–20. Epub 2009/10/22. NEJMoa0908492 [pii] 10.1056/NEJMoa0908492 . - DOI - PubMed
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. Epub 2012/04/06. 10.1056/NEJMoa1113425 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

