Measurement of spinal cord atrophy using phase sensitive inversion recovery (PSIR) imaging in motor neuron disease
- PMID: 30496320
- PMCID: PMC6264489
- DOI: 10.1371/journal.pone.0208255
Measurement of spinal cord atrophy using phase sensitive inversion recovery (PSIR) imaging in motor neuron disease
Abstract
Background: The spectrum of motor neuron disease (MND) includes numerous phenotypes with various life expectancies. The degree of upper and lower motor neuron involvement can impact prognosis. Phase sensitive inversion recovery (PSIR) imaging has been shown to detect in vivo gray matter (GM) and white matter (WM) atrophy in the spinal cord of other patient populations but has not been explored in MND.
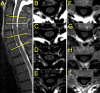
Methods: In this study, total cord, WM and GM areas of ten patients with a diagnosis within the MND spectrum were compared to those of ten healthy controls (HC). Patients' diagnosis included amyotrophic lateral sclerosis (ALS), primary lateral sclerosis, primary muscular atrophy, facial onset sensory and motor neuronopathy and ALS-Frontotemporal dementia. Axial 2D PSIR images were acquired at four cervical disc levels (C2-C3, C3-C4, C5-C6 and C7-T1) with a short acquisition time (2 minutes) protocol. Total cross-sectional areas (TCA), GM and WM areas were measured using a combination of highly reliable manual and semi-automated methods. Cord areas in MND patients were compared with HC using linear regression analyses adjusted for age and sex. Correlation of WM and GM areas in MND patients was explored to gain insights into underlying atrophy patterns.
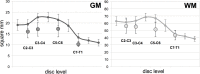
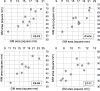
Results: MND patients as a group had significantly smaller cervical cord GM area compared to HC at all four levels (C2-C3: p = .009; C3-C4: p = .001; C5-C6: p = .006; C7-T1: p = .002). WM area at C5-C6 level was significantly smaller (p = .001). TCA was significantly smaller at C3-C4 (p = .018) and C5-C6 (p = .002). No significant GM and WM atrophy was detected in the two patients with predominantly bulbar phenotype. Concomitant GM and WM atrophy was detected in solely upper or lower motor neuron level phenotypes. There was a significant correlation between GM and WM areas at all four levels in this diverse population of MND.
Conclusion: Spinal cord GM and WM atrophy can be detected in vivo in patients within the MND spectrum using a short acquisition time 2D PSIR imaging protocol. PSIR imaging shows promise as a method for quantifying spinal cord involvement and thus may be useful for diagnosis, prognosis and for monitoring disease progression.
Conflict of interest statement
Dr. Miller has served as an Advisor/Director to The Tau Consortium, The John Douglas French Foundation, The Larry L. Hillblom Foundation, Medical Advisory Board, National Institute for Health Research, Cambridge Biomedical Research Centre and its subunit, the Biomedical Research Unit in Dementia (UK); he has served as an External Advisor to University of Washington ADRC, Stanford University ADRC, and University of Pittsburgh ADRC; he receives royalties from Cambridge University Press, Guilford Publications, Inc., and Neurocase. AB reports travel fees from Actelion. NTO has received consulting fees from Avanir pharmaceuticals while participating in the visiting expert program to discuss his prior work on pseudobulbar affect in ALS. All other authors have no conflicts of interest to report. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Kumar DR, Aslinia F, Yale SH, Mazza JJ. Jean-Martin Charcot: the father of neurology. Clin Med Res. 2011;9(1):46–9. 10.3121/cmr.2009.883 ; PubMed Central PMCID: PMCPMC3064755. - DOI - PMC - PubMed
-
- Saberi S, Stauffer JE, Schulte DJ, Ravits J. Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants. Neurol Clin. 2015;33(4):855–76. 10.1016/j.ncl.2015.07.012 ; PubMed Central PMCID: PMCPMC4628785. - DOI - PMC - PubMed
-
- Ravits J, Appel S, Baloh RH, Barohn R, Brooks BR, Elman L, et al. Deciphering amyotrophic lateral sclerosis: what phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14 Suppl 1:5–18. 10.3109/21678421.2013.778548 ; PubMed Central PMCID: PMCPMC3779649. - DOI - PMC - PubMed
-
- Vucic S, Stein TD, Hedley-Whyte ET, Reddel SR, Tisch S, Kotschet K, et al. FOSMN syndrome: novel insight into disease pathophysiology. Neurology. 2012;79(1):73–9. 10.1212/WNL.0b013e31825dce13 . - DOI - PubMed
-
- Ziso B, Williams TL, Walters RJ, Jaiser SR, Attems J, Wieshmann UC, et al. Facial Onset Sensory and Motor Neuronopathy: Further Evidence for a TDP-43 Proteinopathy. Case Rep Neurol. 2015;7(1):95–100. 10.1159/000381944 ; PubMed Central PMCID: PMCPMC4448067. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

