Ovulation: Parallels With Inflammatory Processes
- PMID: 30496379
- PMCID: PMC6405411
- DOI: 10.1210/er.2018-00075
Ovulation: Parallels With Inflammatory Processes
Abstract
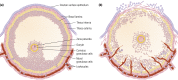
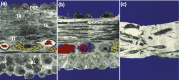
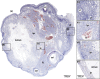
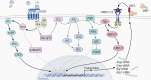
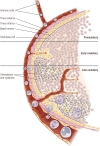
The midcycle surge of LH sets in motion interconnected networks of signaling cascades to bring about rupture of the follicle and release of the oocyte during ovulation. Many mediators of these LH-induced signaling cascades are associated with inflammation, leading to the postulate that ovulation is similar to an inflammatory response. First responders to the LH surge are granulosa and theca cells, which produce steroids, prostaglandins, chemokines, and cytokines, which are also mediators of inflammatory processes. These mediators, in turn, activate both nonimmune ovarian cells as well as resident immune cells within the ovary; additional immune cells are also attracted to the ovary. Collectively, these cells regulate proteolytic pathways to reorganize the follicular stroma, disrupt the granulosa cell basal lamina, and facilitate invasion of vascular endothelial cells. LH-induced mediators initiate cumulus expansion and cumulus oocyte complex detachment, whereas the follicular apex undergoes extensive extracellular matrix remodeling and a loss of the surface epithelium. The remainder of the follicle undergoes rapid angiogenesis and functional differentiation of granulosa and theca cells. Ultimately, these functional and structural changes culminate in follicular rupture and oocyte release. Throughout the ovulatory process, the importance of inflammatory responses is highlighted by the commonalities and similarities between many of these events associated with ovulation and inflammation. However, ovulation includes processes that are distinct from inflammation, such as regulation of steroid action, oocyte maturation, and the eventual release of the oocyte. This review focuses on the commonalities between inflammatory responses and the process of ovulation.
Copyright © 2019 Endocrine Society.
Figures







References
-
- Bukovsky A, Presl J. Ovarian function and the immune system. Med Hypotheses. 1979;5(4):415–436. - PubMed
-
- Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod. 1980;22(1):73–106. - PubMed
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. - PubMed
-
- Espey LL. Ultrastructure of the apex of the rabbit graafian follicle during the ovulatory process. Endocrinology. 1967;81(2):267–276. - PubMed
-
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

