Artificial intelligence-derived 3-Way Concentration-dependent Antagonism of Gatifloxacin, Pyrazinamide, and Rifampicin During Treatment of Pulmonary Tuberculosis
- PMID: 30496458
- PMCID: PMC6904294
- DOI: 10.1093/cid/ciy610
Artificial intelligence-derived 3-Way Concentration-dependent Antagonism of Gatifloxacin, Pyrazinamide, and Rifampicin During Treatment of Pulmonary Tuberculosis
Abstract
Background: In the experimental arm of the OFLOTUB trial, gatifloxacin replaced ethambutol in the standard 4-month regimen for drug-susceptible pulmonary tuberculosis. The study included a nested pharmacokinetic (PK) study. We sought to determine if PK variability played a role in patient outcomes.
Methods: Patients recruited in the trial were followed for 24 months, and relapse ascertained using spoligotyping. Blood was drawn for drug concentrations on 2 separate days during the first 2 months of therapy, and compartmental PK analyses was performed. Failure to attain sustained sputum culture conversion at the end of treatment, relapse, or death during follow-up defined therapy failure. In addition to standard statistical analyses, we utilized an ensemble of machine-learning methods to identify patterns and predictors of therapy failure from among 27 clinical and laboratory features.
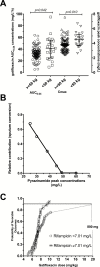
Results: Of 126 patients, 95 (75%) had favorable outcomes and 19 (15%) failed therapy, relapsed, or died. Pyrazinamide and rifampicin peak concentrations and area under the concentration-time curves (AUCs) were ranked higher (more important) than gatifloxacin AUCs. The distribution of individual drug concentrations and their ranking varied significantly between South African and West African trial sites; however, drug concentrations still accounted for 31% and 75% of variance of outcomes, respectively. We identified a 3-way antagonistic interaction of pyrazinamide, gatifloxacin, and rifampicin concentrations. These negative interactions disappeared if rifampicin peak concentration was above 7 mg/L.
Conclusions: Concentration-dependent antagonism contributed to death, relapse, and therapy failure but was abrogated by high rifampicin concentrations. Therefore, increasing both rifampin and gatifloxacin doses could improve outcomes.
Clinical trials registration: NCT00216385.
Figures

References
-
- Rustomjee R, Lienhardt C, Kanyok T, et al. ; Gatifloxacin for TB (OFLOTUB) Study Team. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12:128–38. - PubMed
-
- Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J Infect Dis 2004; 190:1642–51. - PubMed
-
- Alffenaar JW, Gumbo T, Aarnoutse R. Shorter moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2015; 372:576. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

