d-Cycloserine Pharmacokinetics/Pharmacodynamics, Susceptibility, and Dosing Implications in Multidrug-resistant Tuberculosis: A Faustian Deal
- PMID: 30496460
- PMCID: PMC6260153
- DOI: 10.1093/cid/ciy624
d-Cycloserine Pharmacokinetics/Pharmacodynamics, Susceptibility, and Dosing Implications in Multidrug-resistant Tuberculosis: A Faustian Deal
Abstract
Background: d-cycloserine is used to treat multidrug-resistant tuberculosis. Its efficacy, contribution in combination therapy, and best clinical dose are unclear, also data on the d-cycloserine minimum inhibitory concentration (MIC) distributions is scant.
Methods: We performed a systematic search to identify pharmacokinetic and pharmacodynamic studies performed with d-cycloserine. We then performed a combined exposure-effect and dose fractionation study of d-cycloserine in the hollow fiber system model of tuberculosis (HFS-TB). In parallel, we identified d-cycloserine MICs in 415 clinical Mycobacterium tuberculosis (Mtb) isolates from patients. We utilized these results, including intracavitary concentrations, to identify the clinical dose that would be able to achieve or exceed target exposures in 10000 patients using Monte Carlo experiments (MCEs).
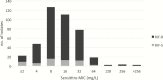
Results: There were no published d-cycloserine pharmacokinetics/pharmacodynamics studies identified. Therefore, we performed new HFS-TB experiments. Cyloserine killed 6.3 log10 colony-forming units (CFU)/mL extracellular bacilli over 28 days. Efficacy was driven by the percentage of time concentration persisted above MIC (%TMIC), with 1.0 log10 CFU/mL kill achieved by %TMIC = 30% (target exposure). The tentative epidemiological cutoff value with the Sensititre MYCOTB assay was 64 mg/L. In MCEs, 750 mg twice daily achieved target exposure in lung cavities of 92% of patients whereas 500 mg twice daily achieved target exposure in 85% of patients with meningitis. The proposed MCE-derived clinical susceptibility breakpoint at the proposed doses was 64 mg/L.
Conclusions: Cycloserine is cidal against Mtb. The susceptibility breakpoint is 64 mg/L. However, the doses likely to achieve the cidality in patients are high, and could be neurotoxic.
Figures



References
-
- Hidy PHH, Hodge EB, Young VV, et al. . Structure and reactions of cycloserine. J Am Chem Soc 1955; 77:2345–6.
-
- Kuehl FA, Wolf FJ, Trenner NR, et al. . D-4-amino-3-isoxazolidinone, a new antibiotic. J Am Chem Soc 1955; 77:2344–5.
-
- Boyd LJ, Epstein IG, Nair KG. The treatment of human tuberculosis with cycloserine: a year’s progress. Antibiot Annu 1955; 3:141–7. - PubMed
-
- Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis 2013; 17:1257–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

