Human mAbs to Staphylococcus aureus IsdA Provide Protection Through Both Heme-Blocking and Fc-Mediated Mechanisms
- PMID: 30496483
- PMCID: PMC6452300
- DOI: 10.1093/infdis/jiy635
Human mAbs to Staphylococcus aureus IsdA Provide Protection Through Both Heme-Blocking and Fc-Mediated Mechanisms
Abstract
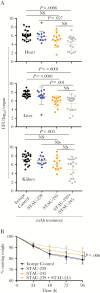
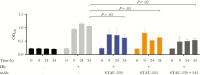
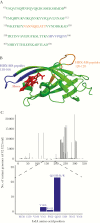
The nutrient metal iron plays a key role in the survival of microorganisms. The iron-regulated surface determinant (Isd) system scavenges heme-iron from the human host, enabling acquisition of iron in iron-deplete conditions in Staphylococcus aureus during infection. The cell surface receptors IsdB and IsdH bind hemoproteins and transfer heme to IsdA, the final surface protein before heme-iron is transported through the peptidoglycan. To define the human B-cell response to IsdA, we isolated human monoclonal antibodies (mAbs) specific to the surface Isd proteins and determined their mechanism of action. We describe the first isolation of fully human IsdA and IsdH mAbs, as well as cross-reactive Isd mAbs. Two of the identified IsdA mAbs worked in a murine septic model of infection to reduce bacterial burden during staphylococcal infection. Their protection was a result of both heme-blocking and Fc-mediated effector functions, underscoring the importance of targeting S. aureus using diverse mechanisms.
Keywords: Staphyloccocus aureus; IsdA; NEAr iron transporter; antibodies; human; iron-regulated surface determinant system.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science 2004; 305:1626–8. - PubMed
-
- Mazmanian SK, Skaar EP, Gaspar AH, et al. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 2003; 299:906–9. - PubMed
-
- Grigg JC, Vermeiren CL, Heinrichs DE, Murphy ME. Haem recognition by a Staphylococcus aureus NEAT domain. Mol Microbiol 2007; 63:139–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

