Excision of uracil from DNA by hSMUG1 includes strand incision and processing
- PMID: 30496516
- PMCID: PMC6344882
- DOI: 10.1093/nar/gky1184
Excision of uracil from DNA by hSMUG1 includes strand incision and processing
Abstract
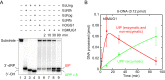
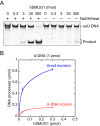
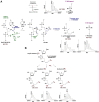
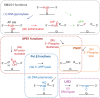
Uracil arises in DNA by hydrolytic deamination of cytosine (C) and by erroneous incorporation of deoxyuridine monophosphate opposite adenine, where the former event is devastating by generation of C → thymine transitions. The base excision repair (BER) pathway replaces uracil by the correct base. In human cells two uracil-DNA glycosylases (UDGs) initiate BER by excising uracil from DNA; one is hSMUG1 (human single-strand-selective mono-functional UDG). We report that repair initiation by hSMUG1 involves strand incision at the uracil site resulting in a 3'-α,β-unsaturated aldehyde designated uracil-DNA incision product (UIP), and a 5'-phosphate. UIP is removed from the 3'-end by human apurinic/apyrimidinic (AP) endonuclease 1 preparing for single-nucleotide insertion. hSMUG1 also incises DNA or processes UIP to a 3'-phosphate designated uracil-DNA processing product (UPP). UIP and UPP were indirectly identified and quantified by polyacrylamide gel electrophoresis and chemically characterised by matrix-assisted laser desorption/ionisation time-of-flight mass-spectrometric analysis of DNA from enzyme reactions using 18O- or 16O-water. The formation of UIP accords with an elimination (E2) reaction where deprotonation of C2' occurs via the formation of a C1' enolate intermediate. A three-phase kinetic model explains rapid uracil excision in phase 1, slow unspecific enzyme adsorption/desorption to DNA in phase 2 and enzyme-dependent AP site incision in phase 3.
Figures






References
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993; 362:709–715. - PubMed
-
- Friedberg E.C., Walker G.C., Siede W., Wood R.D., Schultz R.A., Ellenberger T.. DNA Repair and Mutagenesis. 2006; 2nd ednWashington, DC: ASM Press.
-
- Kornberg A., Baker T.A.. DNA Replication. 1992; 2nd ednNY: W.H. Freeman.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

