Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review
- PMID: 30496988
- PMCID: PMC7111228
- DOI: 10.1016/j.ejmech.2018.11.017
Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review
Abstract
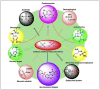
Sulfur (SVI) based moieties, especially, the sulfonyl or sulfonamide based analogues have showed a variety of pharmacological properties, and its derivatives propose a high degree of structural diversity that has established useful for the finding of new therapeutic agents. The developments of new less toxic, low cost and highly active sulfonamides containing analogues are hot research topics in medicinal chemistry. Currently, more than 150 FDA approved Sulfur (SVI)-based drugs are available in the market, and they are widely used to treat various types of diseases with therapeutic power. This comprehensive review highlights the recent developments of sulfonyl or sulfonamides based compounds in huge range of therapeutic applications such as antimicrobial, anti-inflammatory, antiviral, anticonvulsant, antitubercular, antidiabetic, antileishmanial, carbonic anhydrase, antimalarial, anticancer and other medicinal agents. We believe that, this review article is useful to inspire new ideas for structural design and developments of less toxic and powerful Sulfur (SVI) based drugs against the numerous death-causing diseases.
Keywords: Biological activities; Docking; SAR; Sulfonamides; Sulfonyl (S(VI)).
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
Figures
















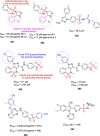
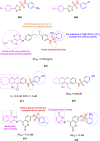
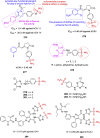
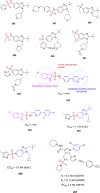

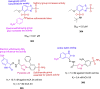

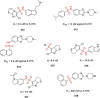
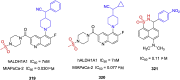

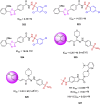


References
-
- Meanwell N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011;54:2529–2591. - PubMed
-
- Majumdar K.C., Mondal S. Recent developments in the synthesis of fused sultams. Chem. Rev. 2011;111:7749–7773. - PubMed
-
- Scozzafava A., Menabuoni L., Mincione F., Briganti F., Mincione G., Supuran C.T. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective, intraocular pressure-lowering aromatic/heterocyclic sulfonamides containing cationic or anionic moieties: is the tail more important than the ring? J. Med. Chem. 1999;42:2641–2650. - PubMed
-
- Stokes S.S., Albert R., Buurman E.T., Andrews B., Shapiro A.B., Green O.M., McKenzie A.R., Otterbein L.R. Inhibitors of the acetyltransferase domain of nacetylglucosamine-1-phosphate-uridylyltransferase/glucosamine-1-phosphate acetyltransferase (GlmU). Part 2: optimization of physical properties leading to antibacterial aryl sulfonamides. Bioorg. Med. Chem. Lett. 2012;22:7019–7023. - PubMed
-
- Konda S., Raparthi S., Bhaskar K., Munaganti R.K., Guguloth V., Nagarapu L., Akkewar D.M. Synthesis and antimicrobial activity of novel benzoxazine sulfonamide derivatives. Bioorg. Med. Chem. Lett. 2015;25:1643–1646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

