GPR119 agonist enhances gefitinib responsiveness through lactate-mediated inhibition of autophagy
- PMID: 30497501
- PMCID: PMC6267899
- DOI: 10.1186/s13046-018-0949-2
GPR119 agonist enhances gefitinib responsiveness through lactate-mediated inhibition of autophagy
Erratum in
-
Correction: GPR119 agonist enhances gefitinib responsiveness through lactate-mediatedinhibition of autophagy.J Exp Clin Cancer Res. 2022 May 17;41(1):176. doi: 10.1186/s13046-022-02388-0. J Exp Clin Cancer Res. 2022. PMID: 35581622 Free PMC article. No abstract available.
Abstract
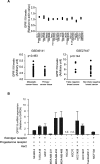
Background: Ligand-dependent activation of the G-protein coupled receptor 119 (GPR119) lowers blood glucose via glucose-dependent insulin secretion and intestinal glucagon-like peptide-1 production. However, the function of GPR119 in cancer cells has not been studied.
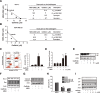
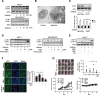
Methods: GPR119 expression was assessed by real-time qPCR and immunohistochemistry in human breast cancer cell lines and breast cancer tissues. Cell proliferation and cell cycle analyses were performed by Incucyte® live cell analysis system and flow cutometry, respectively. Autophagy activity was estimeated by western blottings and LC3-GFP transfection.
Results: mRNA or protein expression of GPR119 was detected in 9 cancer cell lines and 19 tissue samples. Cotreatment with GPR119 agonist (MBX-2982 or GSK1292263) significantly potentiated gefitinib-induced cell growth inhibition in gefitinib-insensitive MCF-7 and MDA-MB-231 breast cancer cells. We observed that caspase-3/7 activity was enhanced with the downregulation of Bcl-2 in MCF-7 cells exposed to MBX-2982. Gefitinib-induced autophagy is related with cancer cell survival and chemoresistance. GPR119 agonists inhibit gefitinib-induced autophagosome formation in MCF-7 and MDA-MB-231 cells. MBX-2982 also caused a metabolic shift to enhanced glycolysis accompanied by reduced mitochondrial oxidative phosphorylation. MBX-2982 increased intracellular (~ 2.5 mM) and extracellular lactate (~ 20 mM) content. Gefitinib-mediated autophagy was suppressed by 20 mM lactate in MCF-7 cells.
Conclusions: GPR119 agonists reduced mitochondrial OXPHOS and stimulated glycolysis in breast cancer cells, with consequent overproduction of lactate that inhibited autophagosome formation. Because autophagy is crucial for the survival of cancer cells exposed to TKIs, GPR119 agonists potentiated the anticancer effects of TKIs.
Keywords: Autophagy; Breast cancer; GPR119 agonist; Gefitinib; Lactate.
Conflict of interest statement
Ethics approval and consent to participate
All human tissues were obtained with patients’ written informed consent and approved by the ethics committee of Chungnam National University. Animal studies were approved by Laboratory Animal Ethics Committee of Seoul National University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

