Effects of Intrauterine Air Bubbles on Embryonic Development in Mice
- PMID: 30497539
- PMCID: PMC6351051
- DOI: 10.30802/AALAS-JAALAS-18-000031
Effects of Intrauterine Air Bubbles on Embryonic Development in Mice
Abstract
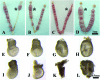
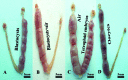
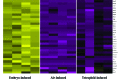
During murine embryo transfer, air bubbles frequently are loaded with embryos into the transfer catheter, but the role of air bubbles on embryonic development is unclear. This study shows that intrauterine air disrupted embryo spacing, induced deciduoma, and impaired postimplantation development. RNA sequencing showed that the gene expression profile of air-induced deciduoma differed significantly from that of embryo-induced decidua but is similar to tetraploid-induced deciduoma. A subset of 33 common genes was upregulated in the embryo-induced decidua compared with air- or tetraploid-induced deciduoma. These data suggest that the inner cell mass (ICM) plays a key role in regulating decidualization and that the trophectoderm is an intermediate that relays ICM-derived signals to other target cells. Our results may provide an innovative approach for detecting the developmental status of embryos in human reproductive medicine.
Figures



References
-
- Bedzhov I, Graham SJ, Leung CY, Zernicka-Goetz M. 2014. Developmental plasticity, cell fate specification, and morphogenesis in the early mouse embryo. Philos Trans R Soc Lond B Biol Sci 369:1–14. https://doi.org/10.1098/rstb.2013.0538 . Correction: 2015. Philos Trans R Soc Lond B Biol Sci 10.1098/rstb.2013.053810.1098/rstb.2014.0339. Correction: 2015. Philos Trans R Soc Lond B Biol Sci https://doi.org/10.1098/rstb.2014.0339 - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

