Ectopic high endothelial venules in pancreatic ductal adenocarcinoma: A unique site for targeted delivery
- PMID: 30497977
- PMCID: PMC6306381
- DOI: 10.1016/j.ebiom.2018.11.030
Ectopic high endothelial venules in pancreatic ductal adenocarcinoma: A unique site for targeted delivery
Abstract
Background: Nanomedicine offers an excellent opportunity to tackle treatment-refractory malignancies by enhancing the delivery of therapeutics to the tumor site. High endothelial venules (HEVs) are found primarily in lymph nodes or formed de novo in peripheral tissues during inflammatory responses. They express peripheral node addressin (PNAd), which is recognized by the monoclonal antibody MECA79.
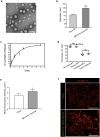
Methods: Here, we demonstrated that HEVs form de novo in human pancreatic ductal adenocarcinoma (PDAC). We engineered MECA79 coated nanoparticles (MECA79-NPs) that recognize these ectopic HEVs in PDAC.
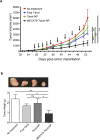
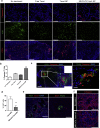
Findings: The trafficking of MECA79-NPs following intravenous delivery to human PDAC implanted in a humanized mouse model was more robust than non-conjugated NPs. Treatment with MECA79-Taxol-NPs augmented the delivery of Paclitaxel (Taxol) to the tumor site and significantly reduced the tumor size. This effect was associated with a higher apoptosis rate of PDAC cells and reduced vascularization within the tumor.
Interpretation: Targeting the HEVs of PDAC using MECA79-NPs could lay the ground for the localized delivery of a wide variety of drugs including chemotherapeutic agents. FUND: National Institutes of Health (NIH) grants: T32-EB016652 (B·B.), NIH Cancer Core Grant CA034196 (L.D.S.), National Institute of Allergy and Infectious Diseases grants R01-AI126596 and R01-HL141815 (R.A.).
Keywords: High endothelial venules; MECA79 coated nanoparticles; Pancreatic ductal adenocarcinoma; Peripheral node addressin; Taxol.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Comment in
-
High Endothelial Venules and Pancreatic Ductal Adenocarcinoma: A potential game changer.EBioMedicine. 2019 Jan;39:29-30. doi: 10.1016/j.ebiom.2018.11.061. Epub 2018 Dec 5. EBioMedicine. 2019. PMID: 30527627 Free PMC article. No abstract available.
References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. - PubMed
-
- Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V. Pancreatic cancer. Nat Rev Dis Prim. 2016;2:16022. - PubMed
-
- Ryan D.P., Hong T.S., Bardeesy N. Pancreatic Adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. - PubMed
-
- Chiaravalli M., Reni M., O'Reilly E.M. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev. 2017;60:32–43. - PubMed

