Genetic recovery of ErbB4 in adulthood partially restores brain functions in null mice
- PMID: 30498032
- PMCID: PMC6304932
- DOI: 10.1073/pnas.1811287115
Genetic recovery of ErbB4 in adulthood partially restores brain functions in null mice
Abstract
Neurotrophic factor NRG1 and its receptor ErbB4 play a role in GABAergic circuit assembly during development. ErbB4 null mice possess fewer interneurons, have decreased GABA release, and show impaired behavior in various paradigms. In addition, NRG1 and ErbB4 have also been implicated in regulating GABAergic transmission and plasticity in matured brains. However, current ErbB4 mutant strains are unable to determine whether phenotypes in adult mutant mice result from abnormal neural development. This important question, a glaring gap in understanding NRG1-ErbB4 function, was addressed by using two strains of mice with temporal control of ErbB4 deletion and expression, respectively. We found that ErbB4 deletion in adult mice impaired behavior and GABA release but had no effect on neuron numbers and morphology. On the other hand, some deficits due to the ErbB4 null mutation during development were alleviated by restoring ErbB4 expression at the adult stage. Together, our results indicate a critical role of NRG1-ErbB4 signaling in GABAergic transmission and behavior in adulthood and suggest that restoring NRG1-ErbB4 signaling at the postdevelopmental stage might benefit relevant brain disorders.
Keywords: ErbB4; GABA; adulthood; schizophrenia; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


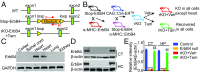

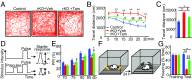
Similar articles
-
ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21818-23. doi: 10.1073/pnas.1010669107. Epub 2010 Nov 24. Proc Natl Acad Sci U S A. 2010. PMID: 21106764 Free PMC article.
-
Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling.Nature. 2010 Apr 29;464(7293):1376-80. doi: 10.1038/nature08928. Epub 2010 Apr 14. Nature. 2010. PMID: 20393464
-
ErbB4 signaling in dopaminergic axonal projections increases extracellular dopamine levels and regulates spatial/working memory behaviors.Mol Psychiatry. 2018 Nov;23(11):2227-2237. doi: 10.1038/mp.2017.132. Epub 2017 Jul 20. Mol Psychiatry. 2018. PMID: 28727685 Free PMC article.
-
Neuregulin 1 in neural development, synaptic plasticity and schizophrenia.Nat Rev Neurosci. 2008 Jun;9(6):437-52. doi: 10.1038/nrn2392. Epub 2008 May 14. Nat Rev Neurosci. 2008. PMID: 18478032 Free PMC article. Review.
-
Neuregulin-1 signalling and antipsychotic treatment: potential therapeutic targets in a schizophrenia candidate signalling pathway.Psychopharmacology (Berl). 2013 Mar;226(2):201-15. doi: 10.1007/s00213-013-3003-2. Epub 2013 Feb 7. Psychopharmacology (Berl). 2013. PMID: 23389757 Review.
Cited by
-
State of the Art in Sub-Phenotyping Midbrain Dopamine Neurons.Biology (Basel). 2024 Sep 3;13(9):690. doi: 10.3390/biology13090690. Biology (Basel). 2024. PMID: 39336117 Free PMC article. Review.
-
Neuregulin 1 and ErbB4 Kinase Actively Regulate Sharp Wave Ripples in the Hippocampus.J Neurosci. 2022 Jan 19;42(3):390-404. doi: 10.1523/JNEUROSCI.1022-21.2021. Epub 2021 Nov 29. J Neurosci. 2022. PMID: 34844988 Free PMC article.
-
ERBB4 exonic deletions on chromosome 2q34 in patients with intellectual disability or epilepsy.Eur J Hum Genet. 2021 Sep;29(9):1377-1383. doi: 10.1038/s41431-021-00815-y. Epub 2021 Feb 18. Eur J Hum Genet. 2021. PMID: 33603162 Free PMC article.
-
Toxic and Phenotypic Effects of AAV_Cre Used to Transduce Mesencephalic Dopaminergic Neurons.Int J Mol Sci. 2022 Aug 21;23(16):9462. doi: 10.3390/ijms23169462. Int J Mol Sci. 2022. PMID: 36012727 Free PMC article.
-
CRISPR/Cas9-mediated Knockout of the Neuropsychiatric Risk Gene KCTD13 Causes Developmental Deficits in Human Cortical Neurons Derived from Induced Pluripotent Stem Cells.Mol Neurobiol. 2020 Feb;57(2):616-634. doi: 10.1007/s12035-019-01727-1. Epub 2019 Aug 11. Mol Neurobiol. 2020. PMID: 31402430
References
-
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. - PubMed
-
- Del Pino I, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. - PubMed
-
- Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. - PubMed
-
- Flames N, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

