Olfactory marker protein (OMP) regulates formation and refinement of the olfactory glomerular map
- PMID: 30498219
- PMCID: PMC6265328
- DOI: 10.1038/s41467-018-07544-9
Olfactory marker protein (OMP) regulates formation and refinement of the olfactory glomerular map
Abstract
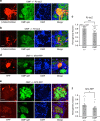
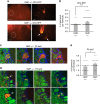
Inputs from olfactory sensory neuron (OSN) axons expressing the same type of odorant receptor (OR) converge in the glomerulus of the main olfactory bulb. A key marker of mature OSNs is olfactory marker protein (OMP), whose deletion has been associated with deficits in OSN signal transduction and odor discrimination. Here, we investigate glomerular odor responses and anatomical architecture in mice in which one or both alleles of OMP are replaced by the fluorescent synaptic activity reporter, synaptopHluorin. Functionally heterogeneous glomeruli, that is, ones with microdomains with distinct odor responses, are rare in OMP+/- mice, but occur frequently in OMP-/- mice. Genetic targeting of single ORs reveals that these microdomains arise from co-innervation of individual glomeruli by OSNs expressing different ORs. This glomerular mistargeting is locally restricted to a few glomerular diameters. Our studies document functional heterogeneity in sensory input within individual glomeruli and uncover its anatomical correlate, revealing an unexpected role for OMP in the formation and refinement of the glomerular map.
Conflict of interest statement
A.C.P. is employed by Cohen Veterans Bioscience, a non-profit public charity research organization. The other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

