Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance
- PMID: 30498240
- PMCID: PMC6561484
- DOI: 10.1038/s10038-018-0542-8
Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance
Abstract
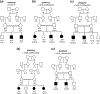
Sinoatrial node dysfunction and deafness (SANDD) syndrome is rare and characterized by a low heart beat and severe-to-profound deafness. Additional features include fatigue, dizziness, and episodic syncope. The sinoatrial node (SAN) drives heart automaticity and continuously regulates heart rate. The CACNA1D gene encoding the Cav1.3 protein expressed in inner hair cells, atria and SAN, induces loss-of-function in channel activity and underlies SANDD. To date, only one variant c.1208_1209insGGG:p.(G403_V404insG) has been reported for SANDD syndrome. We studied five Pakistani families with SANDD and characterized a new missense variant p.(A376V) in CACNA1D in one family, and further characterized the founder variant p.(G403_V404insG) in four additional pedigrees. We show that affected individuals in the four families which segregate p.(G403_V404insG) share a 1.03 MB haplotype on 3p21.1 suggesting they share a common distant ancestor. In conclusion, we identified new and known variants in CACNA1D in five Pakistani families with SANDD. This study is of clinical importance as the CACNA1D founder variant is only observed in families from the Khyber Pakhtunkhwa (KPK) province, in Pakistan. Therefore, screening patients with congenital deafness for SAN dysfunction in this province could ensure adequate follow-up and prevent cardiac failure associated with SAN.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest.
Figures



References
-
- Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med. 2006;354:2151–64. - PubMed
-
- Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, et al. Loss of Ca(v)1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat Neurosci. 2011;14:77–84. - PubMed
-
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DC011651/DC/NIDCD NIH HHS/United States
- R01 DC011651 and R01 DC003594/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
- R01 DC003594/DC/NIDCD NIH HHS/United States
- HHSN268201200008C/HL/NHLBI NIH HHS/United States
- UM1 HG006493/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
