Gold nanorods/siRNA complex administration for knockdown of PARP-1: a potential treatment for perinatal asphyxia
- PMID: 30498346
- PMCID: PMC6207385
- DOI: 10.2147/IJN.S175076
Gold nanorods/siRNA complex administration for knockdown of PARP-1: a potential treatment for perinatal asphyxia
Abstract
Background: Perinatal asphyxia interferes with neonatal development, resulting in long-term deficits associated with systemic and neurological diseases. Despite the important role of poly (ADP-ribose) polymerase 1 (PARP-1) in the regulation of gene expression and DNA repair, overactivation of PARP-1 in asphyxia-exposed animals worsens the ATP-dependent energetic crisis. Inhibition of PARP-1 offers a therapeutic strategy for diminishing the effects of perinatal asphyxia.
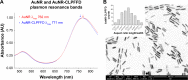
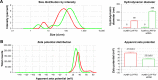
Methods: We designed a nanosystem that incorporates a specific siRNA for PARP-1 knockdown. The siRNA was complexed with gold nanorods (AuNR) conjugated to the peptide CLPFFD for brain targeting.
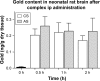
Results: The siRNA was efficiently delivered into PC12 cells, resulting in gene silencing. The complex was administered intraperitoneally in vivo to asphyxia-exposed rat pups, and the ability of the AuNR-CLPFFD/siRNA complex to reach the brain was demonstrated.
Conclusion: The combination of a nanosystem for delivery and a specific siRNA for gene silencing resulted in effective inhibition of PARP-1 in vivo.
Keywords: PARP-1 knockdown; PC12; gold nanorods; in vivo administration; neonatal hypoxia; rats; siRNA delivery.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
-
- Amé JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26(8):882–893. - PubMed
-
- Miwa M, Ida C, Yamashita S, Tanaka M, Fujisawa J. Poly(ADP-ribose): Structure, Physicochemical Properties and Quantification in vivo, with special reference to poly(ADP-ribose) binding protein modules. Curr Protein Pept Sci. 2016;17(7):683–692. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

