Molecular Genetics of the Human Neutrophil Antigens
- PMID: 30498408
- PMCID: PMC6257083
- DOI: 10.1159/000491031
Molecular Genetics of the Human Neutrophil Antigens
Abstract
Background and objective: Antibodies to human neutrophil antigens (HNAs) have been implicated in transfusion-related acute lung injury and allo- and autoimmune neutropenia. To date, five HNA systems are assigned, and during the last decades enormous efforts have been undertaken to identify the underlying genes and to characterize the antigens. This review of the literature will provide the current genetic, molecular and functional information on HNAs.
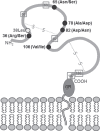
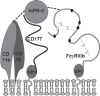
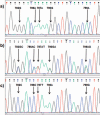
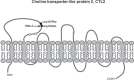
Recent findings: New information on alleles and antigens has been added to nearly each of the five HNA systems. HNA-1d has been added as the antithetical epitope to HNA-1c that is located on the glycoprotein encoded by FCGR3B*02 but not by FCGR3B. FCGR3B*04 and *05 now are included as new alleles. A CD177*787A>T substitution was demonstrated as the main reason for the HNA-2-negative phenotype on neutrophils. The target glycoprotein of HNA-3 antibodies could be identified as choline transporter-like protein 2 (CTL2) encoded by SLC44A2. The conformation sensitive epitope discriminates between arginine and glutamine at position 152 resulting in HNA-3a and HNA-3b. An additional Leu151Phe substitution can impair HNA-3a antibody binding. Recently an alloantibody against HNA-4b which discriminates from HNA-4a by an Arg61His exchange of the glycoprotein encoded by the ITGAM gene was reported in neonatal alloimmune neutropenia. An update of the current HNA nomenclature based on the new findings was provided in 2016 by the ISBT Granulocyte Immunobiology Working Party nomenclature subcommittee.
Conclusions: The molecular basis of each of the five HNA antigen systems has been decoded during the past decades. This enables reliable molecular typing strategies, antibody detection and specification as well as development of new assays based on recombinant antigens. However, research on HNA alleles, antigens, and antibodies is not finally terminated and also in the future will add new findings.
Keywords: Acute lung injury; Alloimmunization; Antibodies; Antigens; Autoimmunity; DNA; Genetic variation; Genotyping; Granulocytes; HNA; Neutrophil; Polymorphism; Single nucleotide; TRALI.
Figures

References
-
- Bux J. Molecular genetics of granulocyte polymorphisms. Vox Sang. 2000;78((suppl 2)):125–130. - PubMed
-
- Flesch BK for the International Society of Blood Transfusion (ISBT) HNA nomenclature subcommittee Human neutrophil antigens: a nomenclature update based on new alleles and new antigens. ISBT Sci Se. 2015;10((suppl 1)):243–249.
-
- Bux J. Human neutrophil alloantigens. Vox Sang. 2008;94:277–285. - PubMed
-
- Bux J. Nomenclature of granulocyte antigens. Transfusion. 1999;39:662–663. - PubMed
-
- Lalezari P, Nussbaum M, Gelman S, Spaet TH. Neonatal neutropenia due to maternal isoimmunization. Blood. 1960;15:236–243. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

