Differences in Intercellular Communication During Clinical Relapse and Gadolinium-Enhanced MRI in Patients With Relapsing Remitting Multiple Sclerosis: A Study of the Composition of Extracellular Vesicles in Cerebrospinal Fluid
- PMID: 30498433
- PMCID: PMC6249419
- DOI: 10.3389/fncel.2018.00418
Differences in Intercellular Communication During Clinical Relapse and Gadolinium-Enhanced MRI in Patients With Relapsing Remitting Multiple Sclerosis: A Study of the Composition of Extracellular Vesicles in Cerebrospinal Fluid
Abstract
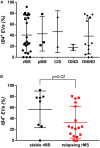
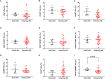
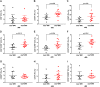
This study was designed based on the hypothesis that changes in both the levels and surface marker expression of extracellular vesicles (EVs) isolated from the cerebrospinal fluid (CSF) may be associated with the clinical form, disease activity, and severity of multiple sclerosis (MS). The analyzes were performed on subjects affected by MS or other neurological disorders. EVs, which were isolated by ultracentrifugation of CSF samples, were characterized by flow cytometry. A panel of fluorescent antibodies was used to identify the EV origin: CD4, CCR3, CCR5, CD19, and CD200, as well as isolectin IB4. The Mann-Whitney U-test and Kruskal-Wallis test were used for statistical analyzes. EVs isolated from the CSF were more abundant in patients with progressive MS and in those with a clinically isolated syndrome than in all the other groups examined. Furthermore, an important change in the number of EVs and in their surface marker expression occurred during active phases of MS [i.e., clinical relapses and the presence of enhancing lesions on magnetic resonance imaging (MRI)]. In particular, the number of CSF-EVs increased in patients affected by MS during clinical relapse; this finding was associated with a decrease in the number of CD19+/CD200+ (naïve B cells) EVs. These markers are expressed by immature and naïve B lymphocytes, and to the best of our knowledge, this double staining has never been associated with MS, but their reduction has been observed in patients with another type of Th1 cell-mediated autoimmune disease. In contrast, the presence of lesions in the brain and spine on gadolinium-enhanced MRI was associated with an increase in the numbers of CCR3+/CCR5+ (subset of CD8 memory T cells), CD4+/CCR3+ (Th2 cells), and CD4+/CCR5+ (Th1 cells) CSF-EVs. Two points are worth emphasizing: (i) the data obtained in this study confirm that CSF-EVs represent a potentially promising tool to identify biomarkers specific for different phases of MS; and (ii) Considering the role of EVs in intercellular communication, our results provide some insights that improve our understanding of the relationships among some of the cell types that are mainly involved in MS pathogenesis (e.g., lymphocytes, glia, and neurons).
Keywords: cerebrospinal fluid; extracellular vesicles; lymphocytes; multiple sclerosis; surface markers.
Figures



References
-
- Babbe H., Roers A., Waisman A., Lassmann H., Goebels N., Hohlfeld R., et al. (2000). Clonal expansions of CD8( + ) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192 393–404. 10.1084/jem.192.3.393 - DOI - PMC - PubMed
-
- Balashov K. E., Rottman J. B., Weiner H. L., Hancock W. W. (1999). CCR5( + ) and CXCR3( + ) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. U. S. A. 96 6873–6878. 10.1073/pnas.96.12.6873 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

