IL-33 Prevents MLD-STZ Induction of Diabetes and Attenuate Insulitis in Prediabetic NOD Mice
- PMID: 30498495
- PMCID: PMC6249384
- DOI: 10.3389/fimmu.2018.02646
IL-33 Prevents MLD-STZ Induction of Diabetes and Attenuate Insulitis in Prediabetic NOD Mice
Abstract
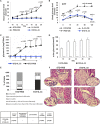
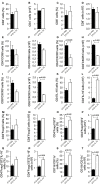
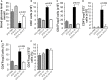
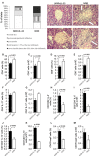
Type 1 diabetes is an autoimmune disease caused by the immune-mediated destruction of pancreatic β-cells. Prevention of type 1 diabetes requires early intervention in the autoimmune process against beta-cells of the pancreatic islets of Langerhans, which is believed to result from disordered immunoregulation. CD4+Foxp3+ regulatory T cells (Tregs) participate as one of the most important cell types in limiting the autoimmune process. The aim of this study was to investigate the effect of exogenous IL-33 in multiple low dose streptozotocin (MLD-STZ) induced diabetes and to delineate its role in the induction of protective Tregs in an autoimmune attack. C57BL/6 mice were treated i. p. with five doses of 40 mg/kg STZ and 0.4 μg rIL-33 four times, starting from day 0, 6, or 12 every second day from the day of disease induction. 16 weeks old NOD mice were treated with 6 injections of 0.4 μg/mouse IL-33 (every second day). Glycemia and glycosuria were measured and histological parameters in pancreatic islets were evaluated at the end of experiments. Cellular make up of the pancreatic lymph nodes and islets were evaluated by flow cytometry. IL-33 given simultaneously with the application of STZ completely prevented the development of hyperglycemia, glycosuria and profoundly attenuated mononuclear cell infiltration. IL-33 treatment was accompanied by higher number of IL-13 and IL-5 producing CD4+ T cells and increased presence of ST2+Foxp3+ regulatory T cells in pancreatic lymph nodes and islets. Elimination of Tregs abrogated protective effect of IL-33. We provide evidence that exogenous IL-33 completely prevents the development of T cell mediated inflammation in pancreatic islets and consecutive development of diabetes in C57BL/6 mice by facilitating the induction Treg cells. To extend this finding for possible relevance in spontaneous diabetes, we showed that IL-33 attenuate insulitis in prediabetic NOD mice.
Keywords: C57BL/6 mice; IL-33; NOD mice; diabetes; streptozotocin.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

