Non-hematopoietic Control of Peripheral Tissue T Cell Responses: Implications for Solid Tumors
- PMID: 30498499
- PMCID: PMC6249380
- DOI: 10.3389/fimmu.2018.02662
Non-hematopoietic Control of Peripheral Tissue T Cell Responses: Implications for Solid Tumors
Abstract
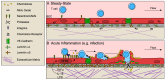
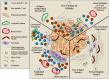
In response to pathological challenge, the host generates rapid, protective adaptive immune responses while simultaneously maintaining tolerance to self and limiting immune pathology. Peripheral tissues (e.g., skin, gut, lung) are simultaneously the first site of pathogen-encounter and also the location of effector function, and mounting evidence indicates that tissues act as scaffolds to facilitate initiation, maintenance, and resolution of local responses. Just as both effector and memory T cells must adapt to their new interstitial environment upon infiltration, tissues are also remodeled in the context of acute inflammation and disease. In this review, we present the biochemical and biophysical mechanisms by which non-hematopoietic stromal cells and extracellular matrix molecules collaborate to regulate T cell behavior in peripheral tissue. Finally, we discuss how tissue remodeling in the context of tumor microenvironments impairs T cell accumulation and function contributing to immune escape and tumor progression.
Keywords: T cell; extravasation; fluid flow; immunotherapy; interstitial migration; non-hematopoietic cells; trafficking.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

