A systematic review of the validity of patient derived xenograft (PDX) models: the implications for translational research and personalised medicine
- PMID: 30498642
- PMCID: PMC6252062
- DOI: 10.7717/peerj.5981
A systematic review of the validity of patient derived xenograft (PDX) models: the implications for translational research and personalised medicine
Abstract
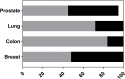
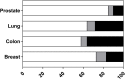
Patient-derived xenograft (PDX) models are increasingly being used in oncology drug development because they offer greater predictive value than traditional cell line models. Using novel tools to critique model validity and reliability we performed a systematic review to identify all original publications describing the derivation of PDX models of colon, prostate, breast and lung cancer. Validity was defined as the ability to recapitulate the disease of interest. The study protocol was registered with the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES). Searches were performed in Embase, MEDLINE and Pubmed up to July 2017. A narrative data synthesis was performed. We identified 105 studies of model validations; 29 for breast, 29 for colon, 25 for lung, 23 for prostate and 4 for multiple tissues. 133 studies were excluded because they did not perform any validation experiments despite deriving a PDX. Only one study reported following the ARRIVE guidelines; developed to improve the standard of reporting for animal experimentation. Remarkably, half of all breast (52%) and prostate (50%) studies were judged to have high concern, in contrast to 16% of colon and 28% of lung studies. The validation criteria that most commonly failed (evidence to the contrary) were: tissue of origin not proven and histology of the xenograft not comparable to the parental tumour. Overall, most studies were categorized as unclear because one or more validation conditions were not reported, or researchers failed to provide data for a proportion of their models. For example, failure to demonstrate tissue of origin, response to standard of care agents and to exclude development of lymphoma. Validation tools have the potential to improve reproducibility, reduce waste in research and increase the success of translational studies.
Keywords: Cancer models; PDX models; Personalised medicine; Systematic review; Validity tools.
Conflict of interest statement
The authors declare there are no competing interests. Shona H. Lang is the co-founder of QED Biomedical, York, UK.
Figures

References
-
- Anderson WC, Boyd MB, Aguilar J, Pickell B, Laysang A, Pysz MA, Bheddah S, Ramoth J, Slingerland BC, Dylla SJ, Rubio ER. Initiation and characterization of small cell lung cancer patient-derived xenografts from ultrasound-guided transbronchial needle aspirates. PLOS ONE. 2015;10:1–13. doi: 10.1371/journal.pone.0125255. - DOI - PMC - PubMed
-
- Ball P. The trouble with scientists. 2015. Nautilus. http://wiki.lib.sun.ac.za/images/d/d7/Trouble-with-scientists.pdf .
LinkOut - more resources
Full Text Sources
Other Literature Sources

