Controlling surface morphology and sensitivity of granular and porous silver films for surface-enhanced Raman scattering, SERS
- PMID: 30498654
- PMCID: PMC6244324
- DOI: 10.3762/bjnano.9.263
Controlling surface morphology and sensitivity of granular and porous silver films for surface-enhanced Raman scattering, SERS
Abstract
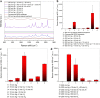
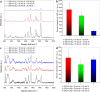
The design of efficient substrates for surface-enhanced Raman spectroscopy (SERS) for large-scale fabrication at low cost is an important issue in further enhancing the use of SERS for routine chemical analysis. Here, we systematically investigate the effect of different radio frequency (rf) plasmas (argon, hydrogen, nitrogen, air and oxygen plasma) as well as combinations of these plasmas on the surface morphology of thin silver films. It was found that different surface structures and different degrees of surface roughness could be obtained by a systematic variation of the plasma type and condition as well as plasma power and treatment time. The differently roughened silver surfaces act as efficient SERS substrates showing greater enhancement factors compared to as prepared, sputtered, but untreated silver films when using rhodamine B as Raman probe molecule. The obtained roughened silver films were fully characterized by scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron (XPS and Auger) and ultraviolet-visible spectroscopy (UV-vis) as well as contact angle measurements. It was found that different morphologies of the roughened Ag films could be obtained under controlled conditions. These silver films show a broad range of tunable SERS enhancement factors ranging from 1.93 × 102 to 2.35 × 105 using rhodamine B as probe molecule. The main factors that control the enhancement are the plasma gas used and the plasma conditions, i.e., pressure, power and treatment time. Altogether this work shows for the first time the effectiveness of a plasma treatment for surface roughening of silver thin films and its profound influence on the interface-controlled SERS enhancement effect. The method can be used for low-cost, large-scale production of SERS substrates.
Keywords: plasma treatment; silver; sputtering; surface roughening; surface-enhanced Raman spectroscopy (SERS).
Figures







Similar articles
-
Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities.Mater Sci Eng C Mater Biol Appl. 2016 Dec 1;69:462-9. doi: 10.1016/j.msec.2016.07.015. Epub 2016 Jul 7. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27612736
-
Silver nanoparticle thin films with nanocavities for surface-enhanced Raman scattering.Chemphyschem. 2008 Apr 21;9(6):902-10. doi: 10.1002/cphc.200800007. Chemphyschem. 2008. PMID: 18366038
-
Tailoring Size and Coverage Density of Silver Nanoparticles on Monodispersed Polymer Spheres as Highly Sensitive SERS Substrates.Chem Asian J. 2016 Sep 6;11(17):2428-35. doi: 10.1002/asia.201600821. Epub 2016 Aug 11. Chem Asian J. 2016. PMID: 27511618
-
Fabrication of surface-enhanced Raman spectroscopy substrates using silver nanoparticles produced by laser ablation in liquids.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Aug 5;296:122694. doi: 10.1016/j.saa.2023.122694. Epub 2023 Apr 5. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 37030254 Review.
-
Beyond the Visible: A Review of Ultraviolet Surface-Enhanced Raman Scattering Substrate Compositions, Morphologies, and Performance.Nanomaterials (Basel). 2023 Jul 26;13(15):2177. doi: 10.3390/nano13152177. Nanomaterials (Basel). 2023. PMID: 37570495 Free PMC article. Review.
Cited by
-
Nanoporous Metals: From Plasmonic Properties to Applications in Enhanced Spectroscopy and Photocatalysis.ACS Nano. 2021 Apr 27;15(4):6038-6060. doi: 10.1021/acsnano.0c10945. Epub 2021 Apr 2. ACS Nano. 2021. PMID: 33797880 Free PMC article.
-
One-Step Etching Characteristics of ITO/Ag/ITO Multilayered Electrode in High-Density and High-Electron-Temperature Plasma.Materials (Basel). 2021 Apr 17;14(8):2025. doi: 10.3390/ma14082025. Materials (Basel). 2021. PMID: 33920632 Free PMC article.
-
Reusable Wrinkled Nanoporous Silver Film Fabricated by Plasma Treatment for Surface-Enhanced Raman Scattering Applications.ACS Omega. 2023 Dec 4;8(49):47146-47152. doi: 10.1021/acsomega.3c07167. eCollection 2023 Dec 12. ACS Omega. 2023. PMID: 38107931 Free PMC article.
References
-
- Sharma B, Frontiera R R, Henry A-I, Ringe E, Van Duyne R P. Mater Today. 2012;15:16–25. doi: 10.1016/s1369-7021(12)70017-2. - DOI
-
- Fleischmann M, Hendra P J, McQuillan A J. Chem Phys Lett. 1974;26:163–166. doi: 10.1016/0009-2614(74)85388-1. - DOI
-
- Albrecht M G, Creighton J A. J Am Chem Soc. 1977;99:5215–5217. doi: 10.1021/ja00457a071. - DOI
-
- Kneipp K. Phys Today. 2007;60:40–46. doi: 10.1063/1.2812122. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
