Protein PEGylation for cancer therapy: bench to bedside
- PMID: 30499020
- PMCID: PMC6732144
- DOI: 10.1007/s12079-018-0492-0
Protein PEGylation for cancer therapy: bench to bedside
Abstract
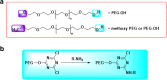
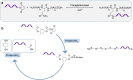
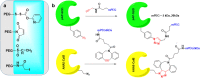
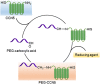
PEGylation is a biochemical modification process of bioactive molecules with polyethylene glycol (PEG), which lends several desirable properties to proteins/peptides, antibodies, and vesicles considered to be used for therapy or genetic modification of cells. However, PEGylation of proteins is a complex process and can be carried out using more than one strategy that depends on the nature of the protein and the desired application. Proteins of interest are covalently conjugated or non-covalently complexed with inert PEG strings. Purification of PEGylated protein is another critical step, which is mainly carried out based on electrostatic interactions or molecular sizes using chromatography. Several PEGylated drugs are being used for diseases like anemia, kidney disease, multiple sclerosis, hemophilia and cancers. With the advancement and increased specificity of the PEGylation process, the world of drug therapy, and specifically cancer therapy could benefit by utilizing this technique to create more stable and non-immunogenic therapies. In this article we describe the structure and functions of PEGylation and how this chemistry helps in drug discovery. Moreover, special emphasis has been given to CCN-family proteins that can be targeted or used as therapy to prevent or block cancer progression through PEGylation technology.
Keywords: Cancer; Immunogenicity; Nanoparticles; PEGylation; Polyethylene glycol.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



References
-
- Abuchowski A, Kazo GM, Verhoest CR, Jr, et al. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys. 1984;7:175–186. - PubMed
-
- Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2002;24:703–729. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

