Extracellular Processing of Lysyl Oxidase-like 2 and Its Effect on Amine Oxidase Activity
- PMID: 30499665
- PMCID: PMC6548334
- DOI: 10.1021/acs.biochem.8b01008
Extracellular Processing of Lysyl Oxidase-like 2 and Its Effect on Amine Oxidase Activity
Abstract
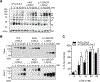
Overexpression of lysyl oxidase-like 2 (LOXL2) is associated with several hepatic and vascular fibrotic diseases and tumor progression in some aggressive cancers. Secreted LOXL2 promotes extracellular matrix cross-linking by catalyzing the oxidative deamination of peptidyl lysine. A great deal remains to be learned about the post-translational modifications of LOXL2, including whether such modifications modulate enzymatic and disease-promoting activities; such knowledge would inform the development of potential therapies. We discovered that upon secretion in cell culture, LOXL2 undergoes proteolytic processing of the first two of four scavenger receptor cysteine-rich domains at the N-terminus. A similar pattern of processing was also evident in tissue extracts from an invasive ductal carcinoma patient. Processing occurred at 314Arg-315Phe-316Arg-317Lys↓-318Ala-, implicating proprotein convertases. siRNA-mediated knockdown of proprotein convertases (furin, PACE4, and PC5/6), as well as incubation with their recombinant forms, showed that PACE4 is the major protease that acts on extracellular LOXL2. Unlike LOX, which requires cleavage of its propeptide for catalytic activation, cleavage of LOXL2 was not essential for tropoelastin oxidation or for cross-linking of collagen type IV in vitro. However, in the latter case, processing enhanced the extent of collagen cross-linking ∼2-fold at ≤10 nM LOXL2. These results demonstrate an important difference in the regulatory mechanisms for LOX and LOXL2 catalytic activity. Moreover, they pave the way for further studies of potential differential functions of LOXL2 isoforms in fibrosis and tumor progression.
Figures




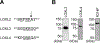

References
-
- Smith-Mungo LI, and Kagan HM (1998) Lysyl oxidase: properties, regulation and multiple functions in biology, Matrix Biol 16, 387–398. - PubMed
-
- Wang SX, Mure M, Medzihradszky KF, Burlingame AL, Brown DE, Dooley DM, Smith AJ, Kagan HM, and Klinman JP (1996) A crosslinked cofactor in lysyl oxidase: redox function for amino acid side chains, Science 273, 1078–1084. - PubMed
-
- Yang J, Savvatis K, Kang JS, Fan P, Zhong H, Schwartz K, Barry V, Mikels-Vigdal A, Karpinski S, Kornyeyev D, Adamkewicz J, Feng X, Zhou Q, Shang C, Kumar P, Phan D, Kasner M, Lopez B, Diez J, Wright KC, Kovacs RL, Chen PS, Quertermous T, Smith V, Yao L, Tschope C, and Chang CP (2016) Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment, Nat Commun 7, 13710. - PMC - PubMed
-
- Kamikawaji K, Seki N, Watanabe M, Mataki H, Kumamoto T, Takagi K, Mizuno K, and Inoue H (2016) Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis, J Hum Genet 61, 985–993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

