Atrial fibrillation and electrophysiology in transgenic mice with cardiac-restricted overexpression of FKBP12
- PMID: 30499712
- PMCID: PMC6397388
- DOI: 10.1152/ajpheart.00486.2018
Atrial fibrillation and electrophysiology in transgenic mice with cardiac-restricted overexpression of FKBP12
Erratum in
-
Corrigendum.Am J Physiol Heart Circ Physiol. 2019 Apr 1;316(4):H938. doi: 10.1152/ajpheart.zh4-2716-corr.2019. Am J Physiol Heart Circ Physiol. 2019. PMID: 30946606 Free PMC article. No abstract available.
Abstract
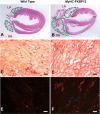
Cardiomyocyte-restricted overexpression of FK506-binding protein 12 transgenic (αMyHC-FKBP12) mice develop spontaneous atrial fibrillation (AF). The aim of the present study is to explore the mechanisms underlying the occurrence of AF in αMyHC-FKBP12 mice. Spontaneous AF was documented by telemetry in vivo and Langendorff-perfused hearts of αMyHC-FKBP12 and littermate control mice in vitro. Atrial conduction velocity was evaluated by optical mapping. The patch-clamp technique was applied to determine the potentially altered electrophysiology in atrial myocytes. Channel protein expression levels were evaluated by Western blot analyses. Spontaneous AF was recorded in four of seven αMyHC-FKBP12 mice but in none of eight nontransgenic (NTG) controls. Atrial conduction velocity was significantly reduced in αMyHC-FKBP12 hearts compared with NTG hearts. Interestingly, the mean action potential duration at 50% but not 90% was significantly prolonged in αMyHC-FKBP12 atrial myocytes compared with their NTG counterparts. Consistent with decreased conduction velocity, average peak Na+ current ( INa) density was dramatically reduced and the INa inactivation curve was shifted by approximately +7 mV in αMyHC-FKBP12 atrial myocytes, whereas the activation and recovery curves were unaltered. The Nav1.5 expression level was significantly reduced in αMyHC-FKBP12 atria. Furthermore, we found increases in atrial Cav1.2 protein levels and peak L-type Ca2+ current density and increased levels of fibrosis in αMyHC-FKBP12 atria. In summary, cardiomyocyte-restricted overexpression of FKBP12 reduces the atrial Nav1.5 expression level and mean peak INa, which is associated with increased peak L-type Ca2+ current and interstitial fibrosis in atria. The combined electrophysiological and structural changes facilitated the development of local conduction block and altered action potential duration and spontaneous AF. NEW & NOTEWORTHY This study addresses a long-standing riddle regarding the role of FK506-binding protein 12 in cardiac physiology. The work provides further evidence that FK506-binding protein 12 is a critical component for regulating voltage-gated sodium current and in so doing has an important role in arrhythmogenic physiology, such as atrial fibrillation.
Keywords: action potential duration; cardiac electrophysiology; fibrosis; ion channels; patch clamp; voltage-gated sodium current.
Conflict of interest statement
Medtronic, St. Jude, Cryocath, and Cyberonics donated research equipment used in this study. P.-S. Chen is a consultant to Cyberonics.
Figures







Similar articles
-
FKBP12 is a critical regulator of the heart rhythm and the cardiac voltage-gated sodium current in mice.Circ Res. 2011 Apr 29;108(9):1042-52. doi: 10.1161/CIRCRESAHA.110.237867. Epub 2011 Mar 3. Circ Res. 2011. PMID: 21372286 Free PMC article.
-
Loss of insulin signaling may contribute to atrial fibrillation and atrial electrical remodeling in type 1 diabetes.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7990-8000. doi: 10.1073/pnas.1914853117. Epub 2020 Mar 20. Proc Natl Acad Sci U S A. 2020. PMID: 32198206 Free PMC article.
-
Exploring SCN5A variants associated with atrial fibrillation in atrial cardiomyocytes derived from human induced pluripotent stem cells: A characterization study.Heart Rhythm. 2025 Jun;22(6):1574-1587. doi: 10.1016/j.hrthm.2024.09.013. Epub 2024 Sep 10. Heart Rhythm. 2025. PMID: 39260661
-
[Biology of the substrate of atrial fibrillation].Biol Aujourdhui. 2012;206(1):5-9. doi: 10.1051/jbio/2012004. Epub 2012 Apr 3. Biol Aujourdhui. 2012. PMID: 22463991 Review. French.
-
Are Interactions between Epicardial Adipose Tissue, Cardiac Fibroblasts and Cardiac Myocytes Instrumental in Atrial Fibrosis and Atrial Fibrillation?Cells. 2021 Sep 21;10(9):2501. doi: 10.3390/cells10092501. Cells. 2021. PMID: 34572150 Free PMC article. Review.
Cited by
-
Hypoxia Promotes Atrial Tachyarrhythmias via Opening of ATP-Sensitive Potassium Channels.Circ Arrhythm Electrophysiol. 2023 Sep;16(9):e011870. doi: 10.1161/CIRCEP.123.011870. Epub 2023 Aug 30. Circ Arrhythm Electrophysiol. 2023. PMID: 37646176 Free PMC article.
-
Dual effect of cardiac FKBP12.6 overexpression on excitation-contraction coupling and the incidence of ventricular arrhythmia depending on its expression level.J Mol Cell Cardiol. 2024 Mar;188:15-29. doi: 10.1016/j.yjmcc.2024.01.003. Epub 2024 Jan 14. J Mol Cell Cardiol. 2024. PMID: 38224852 Free PMC article.
-
Guidelines for assessment of cardiac electrophysiology and arrhythmias in small animals.Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1137-H1166. doi: 10.1152/ajpheart.00439.2022. Epub 2022 Oct 21. Am J Physiol Heart Circ Physiol. 2022. PMID: 36269644 Free PMC article. Review.
-
Lack of authentic atrial fibrillation in commonly used murine atrial fibrillation models.PLoS One. 2022 Jan 7;17(1):e0256512. doi: 10.1371/journal.pone.0256512. eCollection 2022. PLoS One. 2022. PMID: 34995278 Free PMC article.
-
Research Progress of Myocardial Fibrosis and Atrial Fibrillation.Front Cardiovasc Med. 2022 Jul 25;9:889706. doi: 10.3389/fcvm.2022.889706. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35958428 Free PMC article. Review.
References
-
- Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation 119: 606–618, 2009. doi:10.1161/CIRCULATIONAHA.108.825380. - DOI - PMC - PubMed
-
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation 116: 1449–1457, 2007. doi:10.1161/CIRCULATIONAHA.107.704890. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

