The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia
- PMID: 30500073
- PMCID: PMC6467779
- DOI: 10.1002/cncr.31896
The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia
Abstract
Background: Phenotypic characterization of immune cells in the bone marrow (BM) of patients with acute myeloid leukemia (AML) is lacking.
Methods: T-cell infiltration was quantified on BM biopsies from 13 patients with AML, and flow cytometry was performed on BM aspirates (BMAs) from 107 patients with AML who received treatment at The University of Texas MD Anderson Cancer Center. The authors evaluated the expression of inhibitory receptors (programmed cell death protein 1 [PD1], cytotoxic T-lymphocyte antigen 4 [CTLA4], lymphocyte-activation gene 3 [LAG3], T-cell immunoglobulin and mucin-domain containing-3 [TIM3]) and stimulatory receptors (glucocorticoid-induced tumor necrosis factor receptor-related protein [GITR], OX40, 41BB [a type 2 transmembrane glycoprotein receptor], inducible T-cell costimulatory [ICOS]) on T-cell subsets and the expression of their ligands (41BBL, B7-1, B7-2, ICOSL, PD-L1, PD-L2, and OX40L) on AML blasts. Expression of these markers was correlated with patient age, karyotype, baseline next-generation sequencing for 28 myeloid-associated genes (including P53), and DNA methylation proteins (DNA methyltransferase 3α, isocitrate dehydrogenase 1[IDH1], IDH2, Tet methylcytosine dioxygenase 2 [TET2], and Fms-related tyrosine kinase 3 [FLT3]).
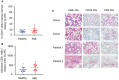
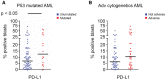
Results: On histochemistry evaluation, the T-cell population in BM appeared to be preserved in patients who had AML compared with healthy donors. The proportion of T-regulatory cells (Tregs) in BMAs was higher in patients with AML than in healthy donors. PD1-positive/OX40-positive T cells were more frequent in AML BMAs, and a higher frequency of PD1-positive/cluster of differentiation 8 (CD8)-positive T cells coexpressed TIM3 or LAG3. PD1-positive/CD8-positive T cells were more frequent in BMAs from patients who had multiply relapsed AML than in BMAs from those who had first relapsed or newly diagnosed AML. Blasts in BMAs from patients who had TP53-mutated AML were more frequently positive for PD-L1.
Conclusions: The preserved T-cell population, the increased frequency of regulatory T cells, and the expression of targetable immune receptors in AML BMAs suggest a role for T-cell-harnessing therapies in AML.
Keywords: T cell; acute myeloid leukemia; flow cytometry; immune checkpoint; immunotherapy.
© 2018 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Conflict of interest statement
Christopher S. Hourigan reports laboratory research funding from Merck and Sellas outside the submitted work. James P. Allison reports consulting fees from and stock ownership in Jounce, Kite Pharma, Neon, Amgen, Forty‐Seven, Apricity, Polaris, Marker Therapeutics, Codiak, BioAlta, ImaginAB, Tvardi Therapeutics, and TapImmune outside the submitted work; patents issued with The Regents of the University of California for “Blockade of Lymphocyte Down‐Regulation Associated With CTLA‐4 Signaling” (US5811097 A, US5855887 A, US6051227 A, and US7229628 B1), “Stimulation of T Cells Against Self Antigens Using CTLA‐4 Blocking Agents” (US20060034844 A1), “Diagnosis of Prostate Cancer with SPAS‐1 Cancer Antigen (US7704701 B2), and “Methods and Compositions for Localized Secretion of Anti‐CTLA‐4 Antibodies” (US9868961 B2); a patent issued with Biosante Pharmaceuticals and The Regents of the University of California for “Cancer Immunotherapy Compositions and Methods of Use” (US7919079 B2); a patient issued with the Icahn School of Medicine at Mount Sinai and Memorial Sloan Kettering Cancer Center for “Newcastle Disease Viruses and Uses Thereof” (US20160015760 A1); a patent issued with the Board of Regents of The University of Texas System and Memorial Sloan Kettering Cancer Center for “Combination Immunotherapy for the Treatment of Cancer (US9375475 B2); a patent issued with Albert Einstein College of Medicine Inc and the Sloan‐Kettering Institute for Cancer Research for “Antibodies to Human B7X for Treatment of Metastatic Cancer” (US9447186 B2); a patent issued for “SPAS‐1 Cancer Antigen (US20020150588 A1); a patent issued for “Compositions and Methods for Modulating Lymphocyte Activity (US20040175380 A1); a patent pending with The Regents of the University of California for “Stimulation of T Cells Against Self Antigens Using CTLA‐4 Blocking Agents” (US20090269353 A1); and a patent pending for “Multi‐Antigen Immunotherapy for Melanoma and Prostate Cancer.” Padmanee Sharma reports consulting fees from and stock ownership in Jounce, Neon, Constellation, Oncolytics, BioAlta, Forty‐Seven, Apricity, Polaris, Marker Therapeutics, and Codiak outside the submitted work and consulting fees from Kite Pharma, Pieris, Merck, and BioMx outside the submitted work. The remaining authors made no disclosures.
Figures



Comment in
-
Will deeper characterization of the landscape of immune checkpoint molecules in acute myeloid leukemia bone marrow lead to improved therapeutic targeting?Cancer. 2019 May 1;125(9):1410-1413. doi: 10.1002/cncr.32042. Epub 2019 Mar 12. Cancer. 2019. PMID: 30861094 Free PMC article.
References
-
- Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280‐3289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

