Activated T cells induce proliferation of oligodendrocyte progenitor cells via release of vascular endothelial cell growth factor-A
- PMID: 30500113
- PMCID: PMC6278606
- DOI: 10.1002/glia.23501
Activated T cells induce proliferation of oligodendrocyte progenitor cells via release of vascular endothelial cell growth factor-A
Abstract
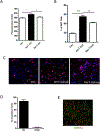
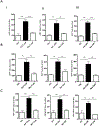
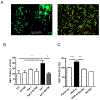
Neuroinflammatory diseases such as multiple sclerosis are characterized by infiltration of lymphocytes into the central nervous system followed by demyelination and axonal degeneration. While evidence suggests that activated T lymphocytes induce neurotoxicity and impair function of neural stem cells, the effect of T cells on oligodendrocyte progenitor cells (OPCs) is still uncertain, partly due to the difficulty in obtaining human OPCs. Here we studied the effect of activated T cells on OPCs using OPCs derived from human hematopoietic stem cells or from human fetal brain. OPCs were exposed to supernatants (sups) from activated T cells. Cell proliferation was determined by EdU incorporation and CellQuanti-Blue assays. Surprisingly, we found that sups from activated T cells induced OPC proliferation by regulating cell cycle progression. Vascular endothelial growth factor A (VEGF-A) transcripts were increased in T cells after activation. Immunodepletion of VEGF-A from activated T cell sups significantly attenuated its effect on OPC proliferation. Furthermore, VEGF receptor 2 (VEGFR2) was expressed on OPCs and its inhibition also attenuated activated T cell-induced OPC proliferation. Thus, activated T cells have a trophic role by promoting OPC proliferation via the VEGFR2 pathway.
Keywords: T cells; VEGF; neural inflammation; neural stem cells; oligodendrocyte progenitor cells.
Published 2018. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Conflict of Interest: The authors declare no competing financial interests.
Figures



References
-
- Al Heialy S, Risse PA, Zeroual MA, Roman HN, Tsuchiya K, Siddiqui S, Laporte SA, Martin JG. 2013. T cell-induced airway smooth muscle cell proliferation via the epidermal growth factor receptor. Am J Respir Cell Mol Biol 49:563–70. - PubMed
-
- Blackburn D, Sargsyan S, Monk PN, Shaw PJ. 2009. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 57:1251–1264. - PubMed
-
- Chandran S, Compston A, Jauniaux E, Gilson J, Blakemore W, Svendsen C. 2004. Differential generation of oligodendrocytes from human and rodent embryonic spinal cord neural precursors. Glia 47:314–324. - PubMed
-
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. 2002. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

