Presentation Mode of Glycans Affect Recognition of Human Serum anti-Neu5Gc IgG Antibodies
- PMID: 30500162
- PMCID: PMC6768799
- DOI: 10.1021/acs.bioconjchem.8b00817
Presentation Mode of Glycans Affect Recognition of Human Serum anti-Neu5Gc IgG Antibodies
Abstract
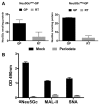
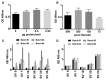
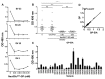
Recognition of carbohydrates by antibodies can be affected by antigen composition and density. This had been investigated in a variety of controllable multivalent systems using synthetic carbohydrate antigens, yet such effects on anticarbohydrate antibodies in circulating human serum have not been fully addressed thus far. All humans develop a polyclonal and diverse response against carbohydrates containing a nonhuman sialic acid form, N-glycolylneuraminic acid (Neu5Gc). This red meat-derived monosaccharide is incorporated into a diverse collection of human glycans resulting in circulating anti-Neu5Gc antibodies in human sera. Such antibodies can cause exacerbation of diseases mediated by chronic inflammation such as cancer and atherosclerosis. We aimed to evaluate how different presentation modes of Neu5Gc-glycans can affect the detection of anti-Neu5Gc IgGs in human serum. Here, we compare serum IgG recognition of Neu5Gc-containing glycoproteins, glycopeptides, and synthetic glycans. First, Neu5Gc-positive or Neu5Gc-deficient mouse strains were used to generate glycopeptides from serum glycoproteins. Then we developed a reproducible ELISA to screen human sera against Neu5Gc-positive glycopeptides for detection of human serum anti-Neu5Gc IgGs. Finally, we evaluated ELISA screens against glycopeptides in comparison with glycoproteins, as well as against elaborated arrays displaying synthetic Neu5Gc-glycans. Our results demonstrate that the presentation mode and diversity of Neu5Gc-glycans are critical for detection of the full collection of human serum anti-Neu5Gc IgGs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Santé study.BMC Med. 2020 Sep 23;18(1):262. doi: 10.1186/s12916-020-01721-8. BMC Med. 2020. PMID: 32962714 Free PMC article.
-
Glycosylated Biotherapeutics: Immunological Effects of N-Glycolylneuraminic Acid.Front Immunol. 2020 Jan 23;11:21. doi: 10.3389/fimmu.2020.00021. eCollection 2020. Front Immunol. 2020. PMID: 32038661 Free PMC article. Review.
-
Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk.PLoS One. 2018 Jun 18;13(6):e0197464. doi: 10.1371/journal.pone.0197464. eCollection 2018. PLoS One. 2018. PMID: 29912879 Free PMC article.
-
Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease.Glycobiology. 2008 Oct;18(10):818-30. doi: 10.1093/glycob/cwn072. Epub 2008 Jul 31. Glycobiology. 2008. PMID: 18669916 Free PMC article.
-
From "Serum Sickness" to "Xenosialitis": Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc.Front Immunol. 2019 Apr 17;10:807. doi: 10.3389/fimmu.2019.00807. eCollection 2019. Front Immunol. 2019. PMID: 31057542 Free PMC article. Review.
Cited by
-
Broad-Spectrum Legionaminic Acid-Specific Antibodies in Pooled Human IgGs Revealed by Glycan Microarrays with Chemoenzymatically Synthesized Nonulosonosides.Molecules. 2024 Aug 22;29(16):3980. doi: 10.3390/molecules29163980. Molecules. 2024. PMID: 39203058 Free PMC article.
-
Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Santé study.BMC Med. 2020 Sep 23;18(1):262. doi: 10.1186/s12916-020-01721-8. BMC Med. 2020. PMID: 32962714 Free PMC article.
-
The Structural Complexity and Animal Tissue Distribution of N-Glycolylneuraminic Acid (Neu5Gc)-Terminated Glycans. Implications for Their Immunogenicity in Clinical Xenografting.Front Mol Biosci. 2019 Jul 19;6:57. doi: 10.3389/fmolb.2019.00057. eCollection 2019. Front Mol Biosci. 2019. PMID: 31428616 Free PMC article. Review.
-
Glycosylated Biotherapeutics: Immunological Effects of N-Glycolylneuraminic Acid.Front Immunol. 2020 Jan 23;11:21. doi: 10.3389/fimmu.2020.00021. eCollection 2020. Front Immunol. 2020. PMID: 32038661 Free PMC article. Review.
-
Serum antibody screening using glycan arrays.Chem Soc Rev. 2024 Mar 4;53(5):2603-2642. doi: 10.1039/d3cs00693j. Chem Soc Rev. 2024. PMID: 38305761 Free PMC article. Review.
References
-
- Varki A, Gagneux P. In: Essentials of Glycobiology. Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2015. - PubMed
-
- Padler-Karavani V. Aiming at the sweet side of cancer: Aberrant glycosylation as possible target for personalized-medicine. Cancer Lett. 2014;352:102–112. - PubMed
-
- Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. - PubMed

