Adolescent development of cortical oscillations: Power, phase, and support of cognitive maturation
- PMID: 30500809
- PMCID: PMC6291169
- DOI: 10.1371/journal.pbio.2004188
Adolescent development of cortical oscillations: Power, phase, and support of cognitive maturation
Abstract
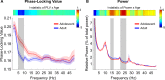
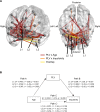
During adolescence, the integration of specialized functional brain networks related to cognitive control continues to increase. Slow frequency oscillations (4-10 Hz) have been shown to support cognitive control processes, especially within prefrontal regions. However, it is unclear how neural oscillations contribute to functional brain network development and improvements in cognitive control during adolescence. To bridge this gap, we employed magnetoencephalography (MEG) to explore changes in oscillatory power and phase coupling across cortical networks in a sample of 68 adolescents and young adults. We found a redistribution of power from lower to higher frequencies throughout adolescence, such that delta band (1-3 Hz) power decreased, whereas beta band power (14-16 and 22-26 Hz) increased. Delta band power decreased with age most strongly in association networks within the frontal lobe and operculum. Conversely, beta band power increased throughout development, most strongly in processing networks and the posterior cingulate cortex, a hub of the default mode (DM) network. In terms of phase, theta band (5-9 Hz) phase-locking robustly decreased with development, following an anterior-to-posterior gradient, with the greatest decoupling occurring between association networks. Additionally, decreased slow frequency phase-locking between frontolimbic regions was related to decreased impulsivity with age. Thus, greater decoupling of slow frequency oscillations may afford functional networks greater flexibility during the resting state to instantiate control when required.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annual Review of Neuroscience. 2015;38: 151–170. 10.1146/annurev-neuro-071714-034054 - DOI - PMC - PubMed
-
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Developmental Science. 2007;10: F8–F14. 10.1111/j.1467-7687.2006.00579.x - DOI - PubMed
-
- Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. The Journal of Neuroscience. 2013;33: 18109–18124. 10.1523/JNEUROSCI.1741-13.2013 - DOI - PMC - PubMed
-
- Crone EA, Zanolie K, Van LL, Westenberg PM, Rombouts SA. Neural mechanisms supporting flexible performance adjustment during development. Cogn AffectBehavNeurosci. 2008;8: 165–177. - PubMed
-
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. Journal of Neuroscience. 2010;30: 15535–15545. 10.1523/JNEUROSCI.2825-10.2010 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

