HIV-1 T cell epitopes targeted to Rhesus macaque CD40 and DCIR: A comparative study of prototype dendritic cell targeting therapeutic vaccine candidates
- PMID: 30500852
- PMCID: PMC6267996
- DOI: 10.1371/journal.pone.0207794
HIV-1 T cell epitopes targeted to Rhesus macaque CD40 and DCIR: A comparative study of prototype dendritic cell targeting therapeutic vaccine candidates
Abstract
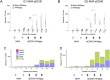
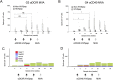
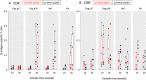
HIV-1 infection can be controlled by anti-retroviral drug therapy, but this is a lifetime treatment and the virus remains latent and rapidly rebounds if therapy is stopped. HIV-1-infected individuals under this drug regimen have increased rates of cancers, cardiovascular diseases, and autoimmunity due to compromised immunity. A therapeutic vaccine boosting cellular immunity against HIV-1 is therefore desirable and, possibly combined with other immune modulating agents, could obviate the need for long-term drug therapies. An approach to elicit strong T cell-based immunity is to direct virus protein antigens specifically to dendritic cells (DCs), which are the key cell type for controlling immune responses. For eliciting therapeutic cellular immunity in HIV-1-infected individuals, we developed vaccines comprised of five T cell epitope-rich regions of HIV-1 Gag, Nef, and Pol (HIV5pep) fused to monoclonal antibodies that bind either, the antigen presenting cell activating receptor CD40, or the endocytic dendritic cell immunoreceptor DCIR. The study aimed to demonstrate vaccine safety, establish efficacy for broad T cell responses in both primed and naïve settings, and identify one candidate vaccine for human therapeutic development. The vaccines were administered to Rhesus macaques by intradermal injection with poly-ICLC adjuvant. The animals were either i) naïve or, ii) previously primed with modified vaccinia Ankara vector (MVA) encoding HIV-1 Gag, Pol, and Nef (MVA GagPolNef). In the MVA-primed groups, both DC-targeting vaccinations boosted HIV5pep-specific blood CD4+ T cells producing multiple cytokines, but did not affect the MVA-elicited CD8+ T cell responses. In the naive groups, both DC-targeting vaccines elicited antigen-specific polyfunctional CD4+ and CD8+ T cell responses to multiple epitopes and these responses were unchanged by a subsequent MVA GagPolNef boost. In both settings, the T cell responses elicited via the CD40-targeting vaccine were more robust and were detectable in all the animals, favoring further development of the CD40-targeting vaccine for therapeutic vaccination of HIV-1-infected individuals.
Conflict of interest statement
All authors have read and approved this manuscript, including the conflict of interest statement, which specifically states: A-L.F., M.M., G.Z., S.Z., and Y.L. are named inventors on patents and patent filings relating to DCIR- and CD40-targeting vaccines that are held jointly by INSERM and the Baylor Research Institute. A.S. holds patents relating to Hiltonol. Relevant patents are: NT07-060/ WO2008097817 Multivariable antigens combined with targeting humanized monoclonal antibody; NT07-086/WO2008097866 Vaccines based on targeting antigen to DCIR expressed on antigen presenting cells; NT09-008/ WO2010104761 Anti-CD40 antibodies and uses thereof; NT09-010/ WO2010104748 Antigen-presenting cell targeted anti-viral vaccines; PCT/US2003/020828 Method for preparation of poly-ICLC and uses thereof; US7834064B2 Clinical method for the immunomodulatory and vaccine adjuvant use of poly-ICLC and other dsRNAs. As noted in the funding statement, one or more of the authors are employed by a commercial company (Advanced BioScience Laboratories, Inc., and Oncovir Inc.). This commercial affiliation does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- Baker BM, Block BL, Rothchild AC, Walker BD. Elite control of HIV infection: implications for vaccine design. Expert Opin Biol Ther. 2009; 9: 55–69. 10.1517/14712590802571928 - DOI - PMC - PubMed
-
- Genovese L, Nebuloni M, Alfano M. Cell-Mediated Immunity in Elite Controllers Naturally Controlling HIV Viral Load. Front Immunol. 2013;4: 86 10.3389/fimmu.2013.00086 - DOI - PMC - PubMed
-
- Chun TW, Davey RT Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401: 874–875. 10.1038/44755 - DOI - PubMed
-
- Pantaleo G, Levy Y. Therapeutic vaccines and immunological intervention in HIV infection: a paradigm change. Curr Opin HIV AIDS. 2016;11(6):576–84. 10.1097/COH.0000000000000324 - DOI - PubMed
-
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113: 6304–6314. 10.1182/blood-2008-10-186601 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

