Defining Changes in the Spatial Distribution and Composition of Brain Lipids in the Shiverer and Cuprizone Mouse Models of Myelin Disease
- PMID: 30501365
- PMCID: PMC6393840
- DOI: 10.1369/0022155418815860
Defining Changes in the Spatial Distribution and Composition of Brain Lipids in the Shiverer and Cuprizone Mouse Models of Myelin Disease
Abstract
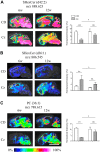
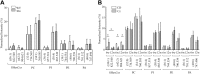
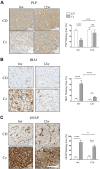
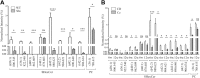
Myelin is composed primarily of lipids and diseases affecting myelin are associated with alterations in its lipid composition. However, correlation of the spatial (in situ) distribution of lipids with the disease-associated compositional and morphological changes is not well defined. Herein we applied high resolution matrix-assisted laser desorption ionization imaging mass spectrometry (MALDI-IMS), immunohistochemistry (IHC), and liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) to evaluate brain lipid alterations in the dysmyelinating shiverer (Shi) mouse and cuprizone (Cz) mouse model of reversible demyelination. MALDI-IMS revealed a decrease in the spatial distribution of sulfatide (SHexCer) species, SHexCer (d42:2), and a phosphatidylcholine (PC) species, PC (36:1), in white matter regions like corpus callosum (CC) both in the Shi mouse and Cz mouse model. Changes in these lipid species were restored albeit not entirely upon spontaneous remyelination after demyelination in the Cz mouse model. Lipid distribution changes correlated with the local morphological changes as confirmed by IHC. LC-ESI-MS analyses of CC extracts confirmed the MALDI-IMS derived reductions in SHexCer and PC species. These findings highlight the role of SHexCer and PC in preserving the normal myelin architecture and our experimental approaches provide a morphological basis to define lipid abnormalities relevant to myelin diseases.
Keywords: brain; cuprizone; electrospray ionization; mass spectrometry; matrix-assisted laser desorption ionization; myelin; shiverer.
Conflict of interest statement
Figures


References
-
- Quarles RH, Macklin WB, Morell P. Myelin formation, structure and biochemistry. In: Brady S, Siegel G, Albers RW, Price D, editors. Basic neurochemistry: molecular, cellular and medical aspects. 7th edition. Burlington: Elsevier Academic Press; 2005. p. 51–71.
-
- Norton WT, Poduso SE. Myelination in rat brain: changes in myelin composition during brain maturation. J Neurochem. 1973;21(4):759–73. - PubMed
-
- Bradl M. Myelin dysfunction/degradation in the central nervous system: why are myelin sheaths susceptible to damage? J Neural Transm Suppl. 1999;55:9–17. - PubMed
-
- Ohler B, Graf K, Bragg R, Lemons T, Coe R, Genain C, Isrealachvili J, Husted C. Role of lipid interactions in autoimmune demyelination. Biochim Biophys Acta. 2004;1688(1):10–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

