Hydroxyurea for Children with Sickle Cell Anemia in Sub-Saharan Africa
- PMID: 30501550
- PMCID: PMC6454575
- DOI: 10.1056/NEJMoa1813598
Hydroxyurea for Children with Sickle Cell Anemia in Sub-Saharan Africa
Abstract
Background: Hydroxyurea is an effective treatment for sickle cell anemia, but few studies have been conducted in sub-Saharan Africa, where the burden is greatest. Coexisting conditions such as malnutrition and malaria may affect the feasibility, safety, and benefits of hydroxyurea in low-resource settings.
Methods: We enrolled children 1 to 10 years of age with sickle cell anemia in four sub-Saharan countries. Children received hydroxyurea at a dose of 15 to 20 mg per kilogram of body weight per day for 6 months, followed by dose escalation. The end points assessed feasibility (enrollment, retention, and adherence), safety (dose levels, toxic effects, and malaria), and benefits (laboratory variables, sickle cell-related events, transfusions, and survival).
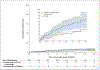
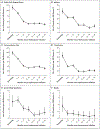
Results: A total of 635 children were fully enrolled; 606 children completed screening and began receiving hydroxyurea at a mean (±SD) dose of 17.5±1.8 mg per kilogram per day. The retention rate was 94.2% at 3 years of treatment. Hydroxyurea therapy led to significant increases in both the hemoglobin and fetal hemoglobin levels. Dose-limiting toxic events regarding laboratory variables occurred in 5.1% of the participants, which was below the protocol-specified threshold for safety. During the treatment phase, 20.6 dose-limiting toxic effects per 100 patient-years occurred, as compared with 20.7 events per 100 patient-years before treatment. As compared with the pretreatment period, the rates of clinical adverse events decreased with hydroxyurea use, including rates of vaso-occlusive pain (98.3 vs. 44.6 events per 100 patient-years; incidence rate ratio, 0.45; 95% confidence interval [CI], 0.37 to 0.56), nonmalaria infection (142.5 vs. 90.0 events per 100 patient-years; incidence rate ratio, 0.62; 95% CI, 0.53 to 0.72), malaria (46.9 vs. 22.9 events per 100 patient-years; incidence rate ratio, 0.49; 95% CI, 0.37 to 0.66), transfusion (43.3 vs. 14.2 events per 100 patient-years; incidence rate ratio, 0.33; 95% CI, 0.23 to 0.47), and death (3.6 vs. 1.1 deaths per 100 patient-years; incidence rate ratio, 0.30; 95% CI, 0.10 to 0.88).
Conclusions: Hydroxyurea treatment was feasible and safe in children with sickle cell anemia living in sub-Saharan Africa. Hydroxyurea use reduced the incidence of vaso-occlusive events, infections, malaria, transfusions, and death, which supports the need for wider access to treatment. (Funded by the National Heart, Lung, and Blood Institute and others; REACH ClinicalTrials.gov number, NCT01966731 .).
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Hydroxyurea - An Essential Medicine for Sickle Cell Disease in Africa.N Engl J Med. 2019 Jan 10;380(2):187-189. doi: 10.1056/NEJMe1814706. N Engl J Med. 2019. PMID: 30625057 No abstract available.
References
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet 2017;390:311–23. - PubMed
-
- Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010;38:Suppl:S512–S521. - PubMed
-
- McGann PT, Nero AC, Ware RE. Clinical features of β-thalassemia and sickle cell disease. Adv Exp Med Biol 2017;1013:1–26. - PubMed
-
- Telfer P, Coen P, Chakravorty S, et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica 2007;92:905–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
