Perioperative multiple low-dose Dexamethasones improves postoperative clinical outcomes after Total knee arthroplasty
- PMID: 30501618
- PMCID: PMC6271578
- DOI: 10.1186/s12891-018-2359-1
Perioperative multiple low-dose Dexamethasones improves postoperative clinical outcomes after Total knee arthroplasty
Abstract
Background: The purpose of this study was to investigate the efficacy and safety of multiple low-dose dexamethasones in primary total knee arthroplasty (TKA).
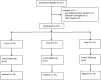
Methods: One hundred fifty patients were equally randomized into 3 groups: Group A (n = 50) received 2 doses of normal saline only; Group B (n = 50) received with 1 dose of intravenous dexamethasone and 1 dose of normal saline; Group C (n = 50) received with 2 doses of intravenous dexamethasone. The clinical outcomes and complications were assessed.
Results: The CRP and IL-6 were significantly lower in Group C and B than Group A at 24, 48, and 72 h postoperatively (P < 0.001 for all). The intensity of postoperative nausea and vomiting (PONV) in Group C was lower than Group A at 24 (P < 0.001, P = 0.002), 48 (P = 0.005, P = 0.041) and 72 h (P = 0.017, P = 0.031) postoperatively and Group B at 24 h (P = 0.027, P = 0.019) postoperatively. Pain were significantly less in Group C than Group A at 24 (P < 0.001), 48 h (P = 0.037) postoperatively and Group B 24 h (P = 0.030) postoperatively. Patients in Group C had better range of motion (ROM) and satisfaction than Group A (P < 0.001, P = 0.002) and B (P = 0.001, P = 0.043). No differences were found in complications.
Conclusions: The administration of 10 mg dexamethasone 1 h before the surgery, and repeated at 6 h postoperatively can significantly reduce the level of postoperative CRP and IL-6 and the incidence of PONV, relieve pain, achieve an additional analgesic effect, and improve the early ROM compared with the other two groups in TKA.
Level of evidence: Therapeutic Level I.
Trial registration: The Chinese Clinical Trial Registry ( ChiCTR1800017036 ). Registered on July 9, 2018.
Keywords: Clinical outcomes; Dexamethasones; Randomized controlled study; Total knee arthroplasty.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of West China Hospital (No: 201302007). All participants will be required to provide written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. - PubMed
-
- Wu Y, Zeng Y, Li C, Zhong J, Hu Q, Pei F, Shen B. The effect of post-operative limb positioning on blood loss and early outcomes after primary total knee arthroplasty: a randomized controlled trial. Int Orthop. Epub 2018 Oct 23. - PubMed
-
- Huang Z, Xie X, Li L, Huang Q, Ma J, Shen B, Kraus VB, Pei F. Intravenous and Topical tranexamic acid alone are superior to tourniquet use for primary Total knee arthroplasty: a prospective, Randomized Controlled Trial. J Bone Joint Surg Am. 2017;99:2053–2061. doi: 10.2106/JBJS.16.01525. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

