Towards a neurobiology of female aggression
- PMID: 30502376
- PMCID: PMC6939635
- DOI: 10.1016/j.neuropharm.2018.11.039
Towards a neurobiology of female aggression
Abstract
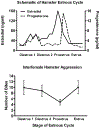
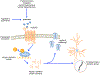
Although many people think of aggression as a negative or undesirable emotion, it is a normal part of many species' repertoire of social behaviors. Purposeful and controlled aggression can be adaptive in that it warns other individuals of perceived breaches in social contracts with the goal of dispersing conflict before it escalates into violence. Aggression becomes maladaptive, however, when it escalates inappropriately or impulsively into violence. Despite ample data demonstrating that impulsive aggression and violence occurs in both men and women, aggression has historically been considered a uniquely masculine trait. As a result, the vast majority of studies attempting to model social aggression in animals, particularly those aimed at understanding the neural underpinnings of aggression, have been conducted in male rodents. In this review, we summarize the state of the literature on the neurobiology of social aggression in female rodents, including social context, hormonal regulation and neural sites of aggression regulation. Our goal is to put historical research in the context of new research, emphasizing studies using ecologically valid methods and modern sophisticated techniques. This article is part of the Special Issue entitled 'Current status of the neurobiology of aggression and impulsivity'.
Keywords: Animal models; Ecological context; Neural circuitry; Sex hormones; Social aggression.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
None declared.
Figures

References
-
- Albert DJ, Dyson EM, Petrovic DM, Walsh ML, 1988. Activation of aggression in female rats by normal males and by castrated males with testosterone implants. Physiol. Behav 44, 9–13. - PubMed
-
- Albert DJ, Jonik RH, Walsh ML, 1993. Influence of combined estradiol and testosterone implants on the aggressiveness of nonaggressive female rats. Physiol. Behav 53, 709–713. - PubMed
-
- Albert DJ, Jonik RH, Walsh ML, 1992. Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neurosci. Biobehav. Rev 16, 177–192. - PubMed
-
- Archer J, 1988. The Behavioural Biology of Aggression. Cambridge: University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

