Oligopeptide Signaling through TbGPR89 Drives Trypanosome Quorum Sensing
- PMID: 30503212
- PMCID: PMC6333907
- DOI: 10.1016/j.cell.2018.10.041
Oligopeptide Signaling through TbGPR89 Drives Trypanosome Quorum Sensing
Abstract
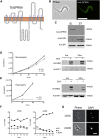
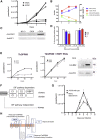
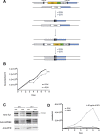
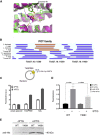
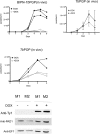
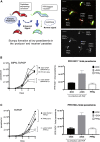
Trypanosome parasites control their virulence and spread by using quorum sensing (QS) to generate transmissible "stumpy forms" in their host bloodstream. However, the QS signal "stumpy induction factor" (SIF) and its reception mechanism are unknown. Although trypanosomes lack G protein-coupled receptor signaling, we have identified a surface GPR89-family protein that regulates stumpy formation. TbGPR89 is expressed on bloodstream "slender form" trypanosomes, which receive the SIF signal, and when ectopically expressed, TbGPR89 drives stumpy formation in a SIF-pathway-dependent process. Structural modeling of TbGPR89 predicts unexpected similarity to oligopeptide transporters (POT), and when expressed in bacteria, TbGPR89 transports oligopeptides. Conversely, expression of an E. coli POT in trypanosomes drives parasite differentiation, and oligopeptides promote stumpy formation in vitro. Furthermore, the expression of secreted trypanosome oligopeptidases generates a paracrine signal that accelerates stumpy formation in vivo. Peptidase-generated oligopeptide QS signals being received through TbGPR89 provides a mechanism for both trypanosome SIF production and reception.
Keywords: GPR89; Trypanosome brucei; differentiation; oligopeptide; parasite; quorum sensing; sleeping sickness; stumpy induction factor.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



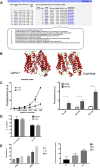

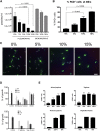
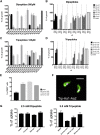
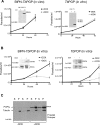

Comment in
-
A Major Step towards Defining the Elusive Stumpy Inducing Factor in Trypanosoma brucei.Trends Parasitol. 2019 Jan;35(1):6-8. doi: 10.1016/j.pt.2018.11.009. Epub 2018 Dec 13. Trends Parasitol. 2019. PMID: 30554967
References
-
- Bangs J.D., Brouch E.M., Ransom D.M., Roggy J.L. A soluble secretory reporter system in Trypanosoma brucei. Studies on endoplasmic reticulum targeting. J. Biol. Chem. 1996;271:18387–18393. - PubMed
-
- Bastin P., Bagherzadeh Z., Matthews K.R., Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol. Biochem. Parasitol. 1996;77:235–239. - PubMed
-
- Bastos I.M., Motta F.N., Charneau S., Santana J.M., Dubost L., Augustyns K., Grellier P. Prolyl oligopeptidase of Trypanosoma brucei hydrolyzes native collagen, peptide hormones and is active in the plasma of infected mice. Microbes Infect. 2010;12:457–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

