Neurobiological and immunogenetic aspects of narcolepsy: Implications for pharmacotherapy
- PMID: 30503715
- PMCID: PMC6351197
- DOI: 10.1016/j.smrv.2018.09.006
Neurobiological and immunogenetic aspects of narcolepsy: Implications for pharmacotherapy
Abstract
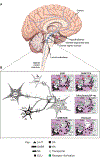
Excessive daytime sleepiness (EDS) and cataplexy are common symptoms of narcolepsy, a sleep disorder associated with the loss of hypocretin/orexin (Hcrt) neurons. Although only a few drugs have received regulatory approval for narcolepsy to date, treatment involves diverse medications that affect multiple biochemical targets and neural circuits. Clinical trials have demonstrated efficacy for the following classes of drugs as narcolepsy treatments: alerting medications (amphetamine, methylphenidate, modafinil/armodafinil, solriamfetol [JZP-110]), antidepressants (tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors), sodium oxybate, and the H3-receptor inverse agonist/antagonist pitolisant. Enhanced catecholamine availability and regulation of locus coeruleus (LC) norepinephrine (NE) neuron activity is likely central to the therapeutic activity of most of these compounds. LC NE neurons are integral to sleep/wake regulation and muscle tone; reduced excitatory input to the LC due to compromise of Hcrt/orexin neurons (likely due to autoimmune factors) results in LC NE dysregulation and contributes to narcolepsy/cataplexy symptoms. Agents that increase catecholamines and/or LC activity may mitigate EDS and cataplexy by elevating NE regulation of GABAergic inputs from the amygdala. Consequently, novel medications and treatment strategies aimed at preserving and/or modulating Hcrt/orexin-LC circuit integrity are warranted in narcolepsy/cataplexy.
Keywords: Amygdala; Autoimmune; Cataplexy; Excessive daytime sleepiness; Hypocretin; Locus coeruleus; Mechanism of action; Narcolepsy; Norepinephrine; Orexin.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of interest
Dr. Szabo has an investigator-initiated grant from Otsuka Pharmaceuticals and funding support from the National Institutes of Health (NIH) and the Brain Behavior Research Foundation (formerly known as NARSAD). He has served on the advisory board for Jazz Pharmaceuticals and as a consultant/speaker for Neurocrine Biosciences, Teva Pharmaceutical Industries Ltd, and Otsuka/Lundbeck Pharmaceuticals.
Dr. Thorpy has received research/grant support and consultancy fees from Jazz Pharmaceuticals, Avadel Pharmaceuticals, Harmony Biosciences, Balance Therapeutics, and Merck Inc.
Dr. Peever has received funding from the National Science and Engineering Research Council and the Canadian Institutes of Health Research.
Dr. Mayer has received honoraria from the Paul Ehrlich Institute, Germany, has served on the speakers’ bureau for UCB Pharma and Sanofi, and is a board member of the European Narcolepsy Network.
Dr. Kilduff has received funding for narcolepsy-related research from the National Institutes of Health, Jazz Pharmaceuticals and TevaBranded Pharmaceuticals Products R&D, Inc.
Figures

References
-
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014; 15: 502–7. - PubMed
-
- Scammell TE. Narcolepsy. N Engl J Med. 2015; 373: 2654–62. - PubMed
-
- Black J, Reaven NL, Funk SE, McGaughey K, Ohayon M, Guilleminault C, et al. The Burden of Narcolepsy Disease (BOND) study: health-care utilization and cost findings. Sleep Med. 2014; 15: 522–9. - PubMed
-
- American_Academy_of_Sleep_Medicine. The International Classification of Sleep Disorders – Third Edition (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

