Drug-associated thrombocytopenia
- PMID: 30504360
- PMCID: PMC6246020
- DOI: 10.1182/asheducation-2018.1.576
Drug-associated thrombocytopenia
Abstract
Many drugs have been implicated in drug-induced immune thrombocytopenia (DITP). Patients with DITP develop a drop in platelet count 5 to 10 days after drug administration with an increased risk of hemorrhage. The diagnosis of DITP is often challenging, because most hospitalized patients are taking multiple medications and have comorbidities that can also cause thrombocytopenia. Specialized laboratory diagnostic tests have been developed and are helpful to confirm the diagnosis. Treatment of DITP involves discontinuation of the offending drug. The platelet count usually starts to recover after 4 or 5 half-lives of the responsible drug or drug metabolite. High doses of intravenous immunoglobulin can be given to patients with severe thrombocytopenia and bleeding. Although in most cases, DITP is associated with bleeding, life-threatening thromboembolic complications are common in patients with heparin-induced thrombocytopenia (HIT). Binding of antiplatelet factor 4/heparin antibodies to Fc receptors on platelets and monocytes causes intravascular cellular activation, leading to an intensely prothrombotic state in HIT. The clinical symptoms include a decrease in platelet counts by >50% and/or new thromboembolic complications. Two approaches can help to confirm or rule out HIT: assessment of the clinical presentation using scoring systems and in vitro demonstration of antiplatelet factor 4/heparin antibodies. The cornerstone of HIT management is immediate discontinuation of heparin when the disease is suspected and anticoagulation using nonheparin anticoagulant. In this review, we will provide an update on the pathophysiology, diagnosis, and management of both DITP and HIT.
© 2018 by The American Society of Hematology. All rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: T.B. has received research grants from the German Society of Research, the German Society for Transfusion Medicine, and the German Red Cross and honoraria from Aspen Germany, CSL Behring, and Stago gGmbH German. I.M. has declared no competing financial interest.
Figures
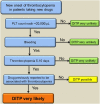

References
-
- Cossu AP, Musu M, Mura P, De Giudici LM, Finco G. Linezolid-induced thrombocytopenia in impaired renal function: is it time for a dose adjustment? A case report and review of literature. Eur J Clin Pharmacol. 2014;70(1):23-28. - PubMed
-
- Erdem GU, Dogan M, Demirci NS, Zengin N. Oxaliplatin-induced acute thrombocytopenia. J Cancer Res Ther. 2016;12(2):509-514. - PubMed
-
- Forcello NP, Khubchandani S, Patel SJ, Brahaj D. Oxaliplatin-induced immune-mediated cytopenias: a case report and literature review. J Oncol Pharm Pract. 2015;21(2):148-156. - PubMed
-
- Mullen TD, Obeid LM. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem. 2012;12(4):340-363. - PubMed
-
- Towhid ST, Tolios A, Münzer P, et al. Stimulation of platelet apoptosis by balhimycin. Biochem Biophys Res Commun. 2013;435(2):323-326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

