Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants
- PMID: 30504857
- PMCID: PMC6269518
- DOI: 10.1038/s41598-018-35850-1
Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of pre-term and full-term human infants
Abstract
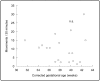
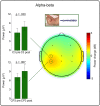
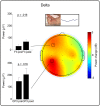
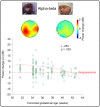
In neonatal animal models, isolated limb movements during active sleep provide input to immature somatomotor cortex necessary for its development and are somatotopically encoded by alpha-beta oscillations as late as the equivalent of human full-term. Limb movements elicit similar neural patterns in very pre-term human infants (average 30 corrected gestational weeks), suggesting an analogous role in humans, but it is unknown until when they subserve this function. In a cohort of 19 neonates (31-42 corrected gestational weeks) we showed that isolated hand movements during active sleep continue to induce these same somatotopically distributed oscillations well into the perinatal period, but that these oscillations decline towards full-term and fully disappear at 41 corrected gestational weeks (equivalent to the end of gestation). We also showed that these highly localised alpha-beta oscillations are associated with an increase in delta oscillations which extends to the frontal area and does not decline with age. These results suggest that isolated limb movements during active sleep could have an important role in experience-dependent somatomotor development up until normal birth in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Emergence of mature cortical activity in wakefulness and sleep in healthy preterm and full-term infants.Sleep. 2018 Aug 1;41(8):zsy096. doi: 10.1093/sleep/zsy096. Sleep. 2018. PMID: 29762768 Free PMC article.
-
Autonomic signatures of late preterm, early term, and full term neonates during early postnatal life.Early Hum Dev. 2019 Oct;137:104817. doi: 10.1016/j.earlhumdev.2019.06.012. Epub 2019 Jul 25. Early Hum Dev. 2019. PMID: 31352221
-
Blood pressure and heart rate patterns during sleep are altered in preterm-born infants: implications for sudden infant death syndrome.Pediatrics. 2008 Dec;122(6):e1242-8. doi: 10.1542/peds.2008-1400. Pediatrics. 2008. PMID: 19047224
-
Normal EEG of premature infants born between 24 and 30 weeks gestational age: terminology, definitions and maturation aspects.Neurophysiol Clin. 2007 Oct-Nov;37(5):311-23. doi: 10.1016/j.neucli.2007.10.008. Epub 2007 Nov 5. Neurophysiol Clin. 2007. PMID: 18063233 Review.
-
The normal EEG of the human newborn.J Clin Neurophysiol. 1985 Apr;2(2):89-103. doi: 10.1097/00004691-198504000-00001. J Clin Neurophysiol. 1985. PMID: 3916842 Review.
Cited by
-
THE DEVELOPING BRAIN REVEALED DURING SLEEP.Curr Opin Physiol. 2020 Jun;15:14-22. doi: 10.1016/j.cophys.2019.11.002. Epub 2019 Nov 18. Curr Opin Physiol. 2020. PMID: 32864534 Free PMC article.
-
Sleep as a window on the sensorimotor foundations of the developing hippocampus.Hippocampus. 2022 Feb;32(2):89-97. doi: 10.1002/hipo.23334. Epub 2021 May 4. Hippocampus. 2022. PMID: 33945190 Free PMC article. Review.
-
Spatiotemporal organization of myoclonic twitching in sleeping human infants.Dev Psychobiol. 2020 Sep;62(6):697-710. doi: 10.1002/dev.21954. Epub 2020 Feb 9. Dev Psychobiol. 2020. PMID: 32037557 Free PMC article.
-
Sleep-wake regulation in preterm and term infants.Sleep. 2021 Jan 21;44(1):zsaa148. doi: 10.1093/sleep/zsaa148. Sleep. 2021. PMID: 32770211 Free PMC article.
-
Fronto-central slow cortical activity is attenuated during phasic events in rapid eye movement sleep at full-term birth.Early Hum Dev. 2019 Sep;136:45-48. doi: 10.1016/j.earlhumdev.2019.07.007. Epub 2019 Jul 11. Early Hum Dev. 2019. PMID: 31302388 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

