D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors
- PMID: 30504927
- PMCID: PMC6372593
- DOI: 10.1038/s41386-018-0286-3
D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors
Abstract
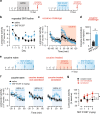
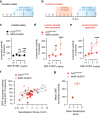
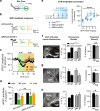
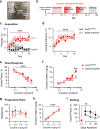
Vulnerability for cocaine abuse in humans is associated with low dopamine D2 receptor (D2R) availability in the striatum. The mechanisms driving this vulnerability are poorly understood. In this study, we found that downregulating D2R expression selectively in striatal indirect-pathway neurons triggers a multitude of changes in D1 receptor (D1R)-expressing direct-pathway neurons, which comprise the other main subpopulation of striatal projection neurons. These changes include a leftward shift in the dose-response to a D1-like agonist that indicates a behavioral D1R hypersensitivity, a shift from PKA to ERK intracellular signaling cascades upon D1R activation, and a reduction in the density of bridging collaterals from D1R-expressing neurons to pallidal areas. We hypothesize that the D1R hypersensitivity underlies abuse vulnerability by facilitating the behavioral responses to repeated cocaine, such as locomotor sensitization and drug self-administration. We found evidence that littermate control mice develop D1R hypersensitivity after they are sensitized to cocaine. Indeed, D1-like agonist and cocaine cross-sensitize in control littermates and this effect was potentiated in mice lacking striatal D2Rs from indirect-pathway neurons. To our surprise, mice with low striatal D2Rs acquired cocaine self-administration similarly to littermate controls and showed no significant change in motivation to take cocaine but lower seeking. These findings indicate that downregulation of striatal D2Rs triggers D1R hypersensitivity to facilitate cocaine locomotor sensitization, which by itself was not associated with greater cocaine taking or seeking under the conditions tested.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

