Seiridium (Sporocadaceae): an important genus of plant pathogenic fungi
- PMID: 30504997
- PMCID: PMC6146642
- DOI: 10.3767/persoonia.2018.40.04
Seiridium (Sporocadaceae): an important genus of plant pathogenic fungi
Abstract
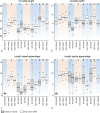
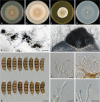
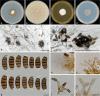
The genus Seiridium includes multiple plant pathogenic fungi well-known as causal organisms of cankers on Cupressaceae. Taxonomically, the status of several species has been a topic of debate, as the phylogeny of the genus remains unresolved and authentic ex-type cultures are mostly absent. In the present study, a large collection of Seiridium cultures and specimens from the CBS and IMI collections was investigated morphologically and phylogenetically to resolve the taxonomy of the genus. These investigations included the type material of the most important Cupressaceae pathogens, Seiridium cardinale, S. cupressi and S. unicorne. We constructed a phylogeny of Seiridium based on four loci, namely the ITS rDNA region, and partial translation elongation factor 1-alpha (TEF), β-tubulin (TUB) and RNA polymerase II core subunit (RPB2). Based on these results we were able to confirm that S. unicorne and S. cupressi represent different species. In addition, five new Seiridium species were described, S. cupressi was lectotypified and epitypes were selected for S. cupressi and S. eucalypti.
Keywords: Cupressus; appendage-bearing conidia; canker pathogen; pestalotioid fungi; systematics.
Figures












References
-
- Ballio A, Castiglione Morelli MA, Evidente A, et al. 1991. Seiricardine A, a phytotoxic sesquiterpene from three Seiridium species pathogenic for cypress. Phytochemistry 30: 131–136.
-
- Barnes I, Roux J, Wingfield MJ, et al. 2001. Characterization of Seiridium spp. associated with cypress canker based on ß-tubulin and Histone sequences. Plant Disease 85: 317–321. - PubMed
-
- Boesewinkel H. 1983. New records of the three fungi causing cypress canker in New Zealand, Seiridium cupressi (Guba) comb. nov. and S. cardinale on Cupressocyparis and S. unicorne on Cryptomeria and Cupressus. Transactions of the British Mycological Society 80: 544–547.
-
- Cho W, Shin H. 2004. List of plant diseases in Korea. Korean Society of Plant Pathology, Seoul, Republic of Korea.
-
- Chou C. 1989. Morphological and cultural variation of Seiridium spp. from cankered Cupressaceae hosts in New Zealand. Forest Pathology 19: 435–445.
LinkOut - more resources
Full Text Sources
