Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe
- PMID: 30504999
- PMCID: PMC6146647
- DOI: 10.3767/persoonia.2018.40.06
Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe
Abstract
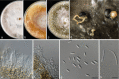
Species of Diaporthe are considered important plant pathogens, saprobes, and endophytes on a wide range of plant hosts. Several species are well-known on grapevines, either as agents of pre- or post-harvest infections, including Phomopsis cane and leaf spot, cane bleaching, swelling arm and trunk cankers. In this study we explore the occurrence, diversity and pathogenicity of Diaporthe spp. associated with Vitis vinifera in major grape production areas of Europe and Israel, focusing on nurseries and vineyards. Surveys were conducted in Croatia, Czech Republic, France, Hungary, Israel, Italy, Spain and the UK. A total of 175 Diaporthe strains were isolated from asymptomatic and symptomatic shoots, branches and trunks. A multi-locus phylogeny was established based on five genomic loci (ITS, tef1, cal, his3 and tub2), and the morphological characters of the isolates were determined. Preliminary pathogenicity tests were performed on green grapevine shoots with representative isolates. The most commonly isolated species were D. eres and D. ampelina. Four new Diaporthe species described here as D. bohemiae, D. celeris, D. hispaniae and D. hungariae were found associated with affected vines. Pathogenicity tests revealed D. baccae, D. celeris, D. hispaniae and D. hungariae as pathogens of grapevines. No symptoms were caused by D. bohemiae. This study represents the first report of D. ambigua and D. baccae on grapevines in Europe. The present study improves our understanding of the species associated with several disease symptoms on V. vinifera plants, and provides useful information for effective disease management.
Keywords: Vitis; canker; multi-locus sequence typing; pathogenicity.
Figures








References
-
- Aveskamp MM, Verkley GJM, De Gruyter J, et al. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101: 363–382. - PubMed
-
- Baumgartner K, Fujiyoshi F, Travadon R, et al. 2013. Characterization of species of Diaporthe from wood cankers of grape in Eastern North American vineyards. Plant Disease 97: 912–920. - PubMed
-
- Carbone I, Kohn LM. 1999. A method for designing primer sets for the speciation studies in filamentous ascomycetes. Mycologia 91: 553–556.
-
- Carroll GC. 1986. The biology of endophytism in plants with particular reference to woody perennials. In: Fokkema NJ, Van den Heuvel J. (eds), Microbiology of the phyllosphere: 205–222. Cambridge University Press, Cambridge.
-
- Chepkirui C, Stadler M. 2017. The genus Diaporthe: a rich source of diverse and bioactive metabolites. Mycological Progress 16: 477–494.
