Fungal Planet description sheets: 716-784
- PMID: 30505003
- PMCID: PMC6146637
- DOI: 10.3767/persoonia.2018.40.10
Fungal Planet description sheets: 716-784
Abstract
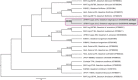
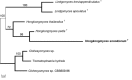
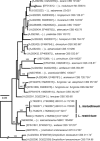
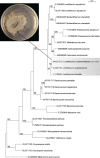
Novel species of fungi described in this study include those from various countries as follows: Australia, Chaetopsina eucalypti on Eucalyptus leaf litter, Colletotrichum cobbittiense from Cordyline stricta × C. australis hybrid, Cyanodermella banksiae on Banksia ericifolia subsp. macrantha, Discosia macrozamiae on Macrozamia miquelii, Elsinoë banksiigena on Banksia marginata, Elsinoë elaeocarpi on Elaeocarpus sp., Elsinoë leucopogonis on Leucopogon sp., Helminthosporium livistonae on Livistona australis, Idriellomyces eucalypti (incl. Idriellomyces gen. nov.) on Eucalyptus obliqua, Lareunionomyces eucalypti on Eucalyptus sp., Myrotheciomyces corymbiae (incl. Myrotheciomyces gen. nov., Myrotheciomycetaceae fam. nov.), Neolauriomyces eucalypti (incl. Neolauriomyces gen. nov., Neolauriomycetaceae fam. nov.) on Eucalyptus sp., Nullicamyces eucalypti (incl. Nullicamyces gen. nov.) on Eucalyptus leaf litter, Oidiodendron eucalypti on Eucalyptus maidenii, Paracladophialophora cyperacearum (incl. Paracladophialophoraceae fam. nov.) and Periconia cyperacearum on leaves of Cyperaceae, Porodiplodia livistonae (incl. Porodiplodia gen. nov., Porodiplodiaceae fam. nov.) on Livistona australis, Sporidesmium melaleucae (incl. Sporidesmiales ord. nov.) on Melaleuca sp., Teratosphaeria sieberi on Eucalyptus sieberi, Thecaphora australiensis in capsules of a variant of Oxalis exilis. Brazil, Aspergillus serratalhadensis from soil, Diaporthe pseudoinconspicua from Poincianella pyramidalis, Fomitiporella pertenuis on dead wood, Geastrum magnosporum on soil, Marquesius aquaticus (incl. Marquesius gen. nov.) from submerged decaying twig and leaves of unidentified plant, Mastigosporella pigmentata from leaves of Qualea parviflorae, Mucor souzae from soil, Mycocalia aquaphila on decaying wood from tidal detritus, Preussia citrullina as endophyte from leaves of Citrullus lanatus, Queiroziella brasiliensis (incl. Queiroziella gen. nov.) as epiphytic yeast on leaves of Portea leptantha, Quixadomyces cearensis (incl. Quixadomyces gen. nov.) on decaying bark, Xylophallus clavatus on rotten wood. Canada, Didymella cari on Carum carvi and Coriandrum sativum. Chile, Araucasphaeria foliorum (incl. Araucasphaeria gen. nov.) on Araucaria araucana, Aspergillus tumidus from soil, Lomentospora valparaisensis from soil. Colombia, Corynespora pseudocassiicola on Byrsonima sp., Eucalyptostroma eucalyptorum on Eucalyptus pellita, Neometulocladosporiella eucalypti (incl. Neometulocladosporiella gen. nov.) on Eucalyptus grandis × urophylla, Tracylla eucalypti (incl. Tracyllaceae fam. nov., Tracyllalales ord. nov.) on Eucalyptus urophylla. Cyprus, Gyromitra anthracobia (incl. Gyromitra subg. Pseudoverpa) on burned soil. Czech Republic, Lecanicillium restrictum from the surface of the wooden barrel, Lecanicillium testudineum from scales of Trachemys scripta elegans. Ecuador, Entoloma yanacolor and Saproamanita quitensis on soil. France, Lentithecium carbonneanum from submerged decorticated Populus branch. Hungary, Pleuromyces hungaricus (incl. Pleuromyces gen. nov.) from a large Fagus sylvatica log. Iran, Zymoseptoria crescenta on Aegilops triuncialis. Malaysia, Ochroconis musicola on Musa sp. Mexico, Cladosporium michoacanense from soil. New Zealand , Acrodontium metrosideri on Metrosideros excelsa, Polynema podocarpi on Podocarpus totara, Pseudoarthrographis phlogis (incl. Pseudoarthrographis gen. nov.) on Phlox subulata. Nigeria, Coprinopsis afrocinerea on soil. Pakistan, Russula mansehraensis on soil under Pinus roxburghii. Russia, Baorangia alexandri on soil in deciduous forests with Quercus mongolica. South Africa, Didymocyrtis brachylaenae on Brachylaena discolor. Spain, Alfaria dactylis from fruit of Phoenix dactylifera, Dothiora infuscans from a blackened wall, Exophiala nidicola from the nest of an unidentified bird, Matsushimaea monilioides from soil, Terfezia morenoi on soil. United Arab Emirates, Tirmania honrubiae on soil. USA, Arxotrichum wyomingense (incl. Arxotrichum gen. nov.) from soil, Hongkongmyces snookiorum from submerged detritus from a fresh water fen, Leratiomyces tesquorum from soil, Talaromyces tabacinus on leaves of Nicotiana tabacum. Vietnam, Afroboletus vietnamensis on soil in an evergreen tropical forest, Colletotrichum condaoense from Ipomoea pes-caprae. Morphological and culture characteristics along with DNA barcodes are provided.
Keywords: ITS nrDNA barcodes; LSU; new taxa; systematics.
Figures













































































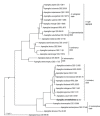
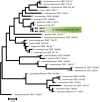


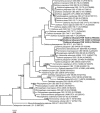

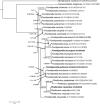

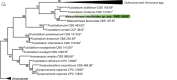

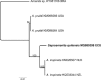
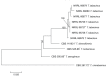
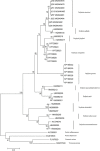




References
-
- Adamčík S, Caboň M, Eberhardt U, et al. 2016. A molecular analysis reveals hidden species diversity within the current concept of Russula maculata (Russulaceae, Basidiomycota). Phytotaxa 270: 71–88.
-
- Álvarez E, Cano J, Stchigel AM, et al. 2011. Two new species of Mucor from clinical samples. Medical Mycology 49: 62–72. - PubMed
-
- Anonymous. 1964. ISCC-NBS centroid color charts. U.S. Department of Commerce, National Bureau of Standards, Washington.
-
- Aptroot A. 2006. Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1–231.
-
- Bates ST. 2004. Arizona members of the Geastraceae and Lycoperdaceae (Basidiomycota, Fungi). Master Thesis, Arizona State University, USA.
LinkOut - more resources
Full Text Sources
